Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.freeradbiomed.2019.12.015 Terrin L Manes 1 , Ari Simenauer 1 , Jason L Geohring 1 , Juliana Flemming 1 , Michael Brehm 2 , Adela Cota-Gomez 1
|
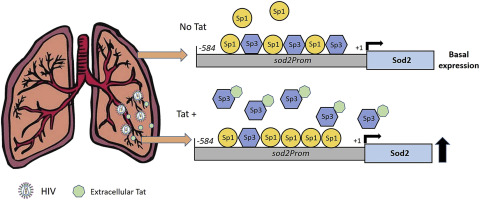
Redox imbalance results in damage to cellular macromolecules and interferes with signaling pathways, leading to an inflammatory cellular and tissue environment. As such, the cellular oxidative environment is tightly regulated by several redox-modulating pathways. Many viruses have evolved intricate mechanisms to manipulate these pathways for their benefit, including HIV-1, which requires a pro-oxidant cellular environment for optimal replication. One such virulence factor responsible for modulating the redox environment is the HIV Transactivator of transcription (Tat). Tat is of particular interest as it is actively secreted by infected cells and internalized by uninfected bystander cells where it can elicit pro-oxidant effects resulting in inflammation and damage. Previously, we demonstrated that Tat regulates basal expression of Superoxide Dismutase 2 (sod2) by altering the binding of the Sp-transcription factors at regions relatively near (approx. −210 nucleotides) upstream of the transcriptional start site. Now, using in silico analysis and a series of sod2 promoter reporter constructs, we have identified putative clusters of Sp-binding sites located further upstream of the proximal sod2 promoter, between nucleotides −3400 to −210, and tested their effect on basal transcription and for their sensitivity to HIV-1 Tat. In this report, we demonstrate that under basal conditions, maximal transcription requires a cluster of Sp-binding sites in the −584 nucleotide region, which is extremely sensitive to Tat. Using chromatin immunoprecipitation (ChIP) we demonstrate that Tat results in altered occupancy of Sp1 and Sp3 at this distal Tat-sensitive regulatory element and strongly stimulated endogenous expression of SOD2 in human pulmonary artery endothelial cells (HPAEC). We also report altered expression of Sp1 and Sp3 in Tat-expressing HPAEC as well as in the lungs of HIV-1 infected humanized mice. Lastly, Tat co-immunoprecipitated with endogenous Sp3 but not Sp1 and did not alter the acetylation state of Sp3. Thus, here, we have defined a novel and important cis-acting factor in HIV-1 Tat-mediated regulation of SOD2, demonstrated that modulation of Sp1 and Sp3 activity by Tat promotes SOD2 expression in primary human pulmonary artery endothelial cells and determined that pulmonary levels of Sp3 as well as SOD2 are increased in the lungs of a mouse model of HIV infection.
中文翻译:

HIV-Tat 蛋白与 Sp3 转录因子相互作用并抑制其与人肺动脉内皮细胞中 sod2 启动子远端位点的结合。
氧化还原失衡导致细胞大分子受损并干扰信号通路,导致炎症细胞和组织环境。因此,细胞氧化环境受到几种氧化还原调节途径的严格调节。许多病毒已经进化出复杂的机制来操纵这些途径以获得它们的利益,包括 HIV-1,它需要一个促氧化细胞环境才能实现最佳复制。一种负责调节氧化还原环境的毒力因子是 HIV 转录反式激活因子 (Tat)。Tat 特别令人感兴趣,因为它由受感染的细胞积极分泌,并被未感染的旁观者细胞内化,在那里它可以引发促氧化作用,从而导致炎症和损伤。之前,sod2)通过改变转录起始位点上游相对靠近(约 -210 个核苷酸)区域的 Sp 转录因子的结合。现在,使用计算机分析和一系列sod2启动子报告结构,我们已经确定了位于近端sod2上游更远的 Sp 结合位点的假定簇启动子,在核苷酸-3400到-210之间,并测试了它们对基础转录的影响以及它们对HIV-1 Tat的敏感性。在本报告中,我们证明在基础条件下,最大转录需要 -584 核苷酸区域中的一组 Sp 结合位点,这对 Tat 极为敏感。使用染色质免疫沉淀 (ChIP),我们证明 Tat 会改变这个远端 Tat 敏感调节元件处 Sp1 和 Sp3 的占有率,并强烈刺激人肺动脉内皮细胞 (HPAEC) 中 SOD2 的内源性表达。我们还报告了表达 Tat 的 HPAEC 以及 HIV-1 感染的人源化小鼠肺中 Sp1 和 Sp3 的表达改变。最后,Tat 与内源性 Sp3 而不是 Sp1 共免疫沉淀,并且没有改变 Sp3 的乙酰化状态。因此,在这里,











































 京公网安备 11010802027423号
京公网安备 11010802027423号