当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative misfolding of Cu/Zn-superoxide dismutase triggered by non-canonical intramolecular disulfide formation.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.freeradbiomed.2019.12.017 Itsuki Anzai 1 , Eiichi Tokuda 1 , Sumika Handa 1 , Hidemi Misawa 2 , Shuji Akiyama 3 , Yoshiaki Furukawa 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2019-12-19 , DOI: 10.1016/j.freeradbiomed.2019.12.017 Itsuki Anzai 1 , Eiichi Tokuda 1 , Sumika Handa 1 , Hidemi Misawa 2 , Shuji Akiyama 3 , Yoshiaki Furukawa 1
Affiliation
|
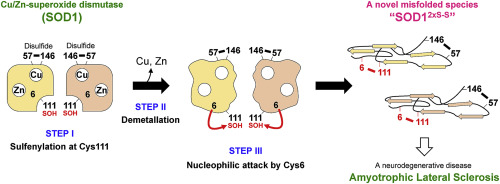
Misfolded Cu/Zn-superoxide dismutase (SOD1) is a pathological species in a subset of amyotrophic lateral sclerosis (ALS). Oxidative stress is known to increase in affected spinal cords of ALS and is thus considered to cause damages on SOD1 leading to the misfolding and aggregation. Despite this, it still remains elusive what triggers misfolding of SOD1 under oxidizing environment. Here, we show that a thiol group of Cys111 in SOD1 is oxidized to a sulfenic acid with hydrogen peroxide and reveal that further dissociation of the bound metal ions from the oxidized SOD1 allows another free Cys residue (Cys6) to nucleophilically attack the sulfenylated Cys111. As a result, an intra-molecular disulfide bond forms between Cys6 and Cys111. Such an abnormal SOD1 with the non-canonical disulfide bond was conformationally extended with significant cytotoxicity as well as high propensity to aggregate. Taken together, we propose a new model of SOD1 misfolding under oxidizing environment, in which formation of the non-canonical intramolecular disulfide bond plays a pivotal role.
中文翻译:

非规范的分子内二硫键形成触发的铜/锌超氧化物歧化酶的氧化错误折叠。
错折叠的铜/锌超氧化物歧化酶(SOD1)是肌萎缩性侧索硬化(ALS)子集中的一种病理物种。已知氧化应激会在受影响的ALS脊髓中增加,因此被认为会对SOD1造成损害,从而导致错误折叠和聚集。尽管如此,在氧化环境下触发SOD1错折叠的原因仍然难以捉摸。在这里,我们显示SOD1中Cys111的硫醇基团被过氧化氢氧化为亚硫酸,并揭示了结合的金属离子从氧化后的SOD1进一步解离使另一个游离的Cys残基(Cys6)亲核性攻击了亚硫酰化的Cys111。结果,在Cys6和Cys111之间形成了分子内二硫键。具有非典型二硫键的这种异常SOD1在构象上扩展,具有明显的细胞毒性以及极高的聚集倾向。综上所述,我们提出了一种在氧化环境下SOD1错折叠的新模型,其中非典型分子内二硫键的形成起着关键作用。
更新日期:2019-12-19
中文翻译:

非规范的分子内二硫键形成触发的铜/锌超氧化物歧化酶的氧化错误折叠。
错折叠的铜/锌超氧化物歧化酶(SOD1)是肌萎缩性侧索硬化(ALS)子集中的一种病理物种。已知氧化应激会在受影响的ALS脊髓中增加,因此被认为会对SOD1造成损害,从而导致错误折叠和聚集。尽管如此,在氧化环境下触发SOD1错折叠的原因仍然难以捉摸。在这里,我们显示SOD1中Cys111的硫醇基团被过氧化氢氧化为亚硫酸,并揭示了结合的金属离子从氧化后的SOD1进一步解离使另一个游离的Cys残基(Cys6)亲核性攻击了亚硫酰化的Cys111。结果,在Cys6和Cys111之间形成了分子内二硫键。具有非典型二硫键的这种异常SOD1在构象上扩展,具有明显的细胞毒性以及极高的聚集倾向。综上所述,我们提出了一种在氧化环境下SOD1错折叠的新模型,其中非典型分子内二硫键的形成起着关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号