当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational and Experimental Study of Fluorine Doped (Mn1–xNbx)O2 Nanorod Electrocatalysts for Acid-Mediated Oxygen Evolution Reaction
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-18 00:00:00 , DOI: 10.1021/acsaem.9b01796 Shrinath Dattatray Ghadge 1 , Oleg I. Velikokhatnyi 2, 3 , Moni K. Datta 2, 3 , Pavithra M. Shanthi 1 , Susheng Tan 4 , Prashant N. Kumta 1, 2, 3, 5, 6
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-12-18 00:00:00 , DOI: 10.1021/acsaem.9b01796 Shrinath Dattatray Ghadge 1 , Oleg I. Velikokhatnyi 2, 3 , Moni K. Datta 2, 3 , Pavithra M. Shanthi 1 , Susheng Tan 4 , Prashant N. Kumta 1, 2, 3, 5, 6
Affiliation
|
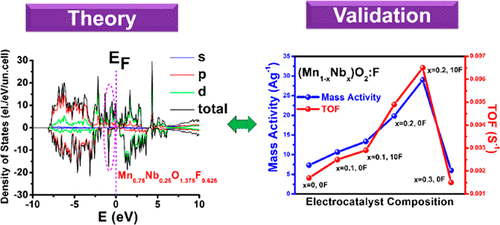
Identification, development, and engineering of high-performance, earth-abundant, and cost-effective precious group metal (PGM)-free electrocatalysts for catalyzing oxygen evolution reaction (OER) in acidic electrolytes are vital for the commercialization of proton exchange membrane based water electrolysis (PEMWE) technology. Utilizing the density functional theory (DFT) calculations to rationalize the thermodynamics and kinetics of adsorption of OER, juxtaposed with cohesive energy and electronic structure, we report the generation of 10 wt % fluorine (F)-doped (Mn1–xNbx)O2:10F nanorods (NRs) as active and durable PGM-free solid solution electrocatalysts for acid-mediated OER. The DFT calculations reveal an optimal solid solution composition of (Mn0.8Nb0.2)O2:10F containing Nb and F in α-MnO2 structure, exhibiting the optimized surface electronic structure (ΔG for the OER rate-determining step ∼ 1.72 eV) and cohesive energy (Ecoh ∼ −16.30 eV/(formula unit)) for OER, contributing to its higher catalytic performance in comparison to α-MnO2. Consequently, (Mn1–xNbx)O2:10F compositions with well-defined one-dimensional (1D) nanorod architectures are synthesized with the optimal composition of (Mn0.8Nb0.2)O2:10F, demonstrating improved electrocatalytic performance for acidic OER in good agreement with the DFT calculations. The superior electrochemical performance of (Mn0.8Nb0.2)O2:10F NRs includes significantly lower charge transfer resistance (∼11.8 Ω cm2), lower Tafel slope (∼371.17 mV dec–1), lower overpotential to deliver a current density of 10 mA cm–2geo (∼0.68 V), higher mass activity (∼29 A g–1), large electrochemically active surface area (ECSA ∼ 26.28 m2g–1), and turnover frequency (TOF ∼ 0.0065 s–1) with higher BET and ECSA normalized activity (∼0.5 mA cm–2BET and 0.11 mA cm–2ECSA) contrasted with (Mn1–xNbx)O2:10F (x = 0, 0.1, and 0.3) compositions, at an overpotential of 0.67 mV. Further, (Mn0.8Nb0.2)O2:10F NRs exhibit good electrochemical stability in acidic OER regimes, with no substantial catalytic activity degradation, validating its structural robustness for prolonged OER and making it a promising PGM-free OER electrocatalyst for acid-mediated PEMWE.
中文翻译:

掺氟(Mn 1– x Nb x)O 2纳米棒电催化剂在酸介导的析氧反应中的计算与实验研究
鉴定,开发和设计高性能,不含土的高性价比高价无金属贵金属(PGM)催化剂,用于催化酸性电解质中的氧释放反应(OER),对于基于质子交换膜的水的商业化至关重要电解(PEMWE)技术。利用密度泛函理论(DFT)计算来合理化OER吸附的热力学和动力学,并结合内聚能和电子结构,我们报告了掺杂10 wt%的氟(F)掺杂(Mn 1– x Nb x)的产生Ø 2:10F纳米棒(NRs)作为活性和耐用的无PGM固溶体电催化剂,用于酸介导的OER。在DFT计算揭示的(锰的最佳固体溶液组合物0.8的Nb 0.2)O 2:含铌和F 10Fα-MnO的2结构中,表现出优化的表面的电子结构(Δ ģ为OER速率决定步骤〜1.72电子伏特)和内聚能(Ë COH〜-16.30电子伏特/(式单元)),用于OER,相比于α-MnO的促进其更高的催化性能2。因此,(Mn 1– x Nb x)O 2合成具有定义明确的一维(1D)纳米棒结构的:10F成分,并以(Mn 0.8 Nb 0.2)O 2:10F的最佳成分合成,证明了酸性OER的改进的电催化性能与DFT计算具有很好的一致性。(Mn 0.8 Nb 0.2)O 2:10F NRs的优异电化学性能包括显着降低的电荷转移电阻(〜11.8Ωcm 2),更低的Tafel斜率(〜371.17 mV dec –1),更低的过电势,可提供的电流密度为10 mA cm –2 geo(〜0.68 V),更高的质量活度(〜29 A g –1),较大的电化学活性表面积(ECSA〜26.28 m 2 g –1)和周转频率(TOF〜0.0065 s –1),具有较高的BET和ECSA归一化活性(〜0.5 mA cm –2 BET和0.11 mA cm –2 ECSA)与(Mn 1- x Nb x)O 2:10F(x = 0、0.1和0.3)组成物形成对比,其超电势为0.67 mV。此外,(Mn 0.8 Nb 0.2)O 2:10F NRs在酸性OER方案中表现出良好的电化学稳定性,而没有实质性的催化活性降解,验证了其对延长OER的结构稳健性,使其成为酸介导的PEMWE的有前途的无PGM的OER电催化剂。
更新日期:2019-12-18
中文翻译:

掺氟(Mn 1– x Nb x)O 2纳米棒电催化剂在酸介导的析氧反应中的计算与实验研究
鉴定,开发和设计高性能,不含土的高性价比高价无金属贵金属(PGM)催化剂,用于催化酸性电解质中的氧释放反应(OER),对于基于质子交换膜的水的商业化至关重要电解(PEMWE)技术。利用密度泛函理论(DFT)计算来合理化OER吸附的热力学和动力学,并结合内聚能和电子结构,我们报告了掺杂10 wt%的氟(F)掺杂(Mn 1– x Nb x)的产生Ø 2:10F纳米棒(NRs)作为活性和耐用的无PGM固溶体电催化剂,用于酸介导的OER。在DFT计算揭示的(锰的最佳固体溶液组合物0.8的Nb 0.2)O 2:含铌和F 10Fα-MnO的2结构中,表现出优化的表面的电子结构(Δ ģ为OER速率决定步骤〜1.72电子伏特)和内聚能(Ë COH〜-16.30电子伏特/(式单元)),用于OER,相比于α-MnO的促进其更高的催化性能2。因此,(Mn 1– x Nb x)O 2合成具有定义明确的一维(1D)纳米棒结构的:10F成分,并以(Mn 0.8 Nb 0.2)O 2:10F的最佳成分合成,证明了酸性OER的改进的电催化性能与DFT计算具有很好的一致性。(Mn 0.8 Nb 0.2)O 2:10F NRs的优异电化学性能包括显着降低的电荷转移电阻(〜11.8Ωcm 2),更低的Tafel斜率(〜371.17 mV dec –1),更低的过电势,可提供的电流密度为10 mA cm –2 geo(〜0.68 V),更高的质量活度(〜29 A g –1),较大的电化学活性表面积(ECSA〜26.28 m 2 g –1)和周转频率(TOF〜0.0065 s –1),具有较高的BET和ECSA归一化活性(〜0.5 mA cm –2 BET和0.11 mA cm –2 ECSA)与(Mn 1- x Nb x)O 2:10F(x = 0、0.1和0.3)组成物形成对比,其超电势为0.67 mV。此外,(Mn 0.8 Nb 0.2)O 2:10F NRs在酸性OER方案中表现出良好的电化学稳定性,而没有实质性的催化活性降解,验证了其对延长OER的结构稳健性,使其成为酸介导的PEMWE的有前途的无PGM的OER电催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号