当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Experimental Study of the Formation of Organosulfates from α-Pinene Oxidation. 2. Time Evolution and Effect of Particle Acidity.
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jpca.9b07156 G Duporté 1, 2 , P-M Flaud 1, 2 , J Kammer 1, 2 , E Geneste 1, 2 , S Augagneur 1, 2 , E Pangui 3 , H Lamkaddam 3 , A Gratien 3 , J-F Doussin 3 , H Budzinski 1, 2 , E Villenave 1, 2 , E Perraudin 1, 2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.jpca.9b07156 G Duporté 1, 2 , P-M Flaud 1, 2 , J Kammer 1, 2 , E Geneste 1, 2 , S Augagneur 1, 2 , E Pangui 3 , H Lamkaddam 3 , A Gratien 3 , J-F Doussin 3 , H Budzinski 1, 2 , E Villenave 1, 2 , E Perraudin 1, 2
Affiliation
|
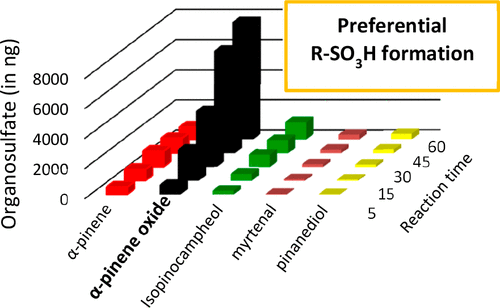
The present work is an extensive laboratory study of organosulfate (OS) formation from the reaction of α-pinene oxidation products or proxies with acidified ammonium sulfate aerosols in three different acidity conditions ((NH4)2SO4 0.06 M; (NH4)2SO4/H2SO4 0.06 M/0.005 M; (NH4)2SO4/H2SO4 0.03 M/0.05 M). The kinetics of the reactions of α-pinene, α-pinene oxide, isopinocampheol, pinanediol, and myrtenal with ammonium sulfate particles were studied using a quasi-static reactor. The reaction of α-pinene oxide with the highly acidic ammonium sulfate particles was determined to be 7, 10, 21, and 24 times faster than for isopinocampheol, α-pinene, pinanedial, and myrtenal, respectively, for an OS precursor concentration of 1 ppm and after 1 h reaction time. The effective rate coefficients for OS formation from α-pinene oxide were determined to be 2 orders of magnitude higher in highly acidic conditions than for the two other acidity conditions. For α-pinene oxide reactions with highly acidic ammonium sulfate particles, OS formation was observed to increase linearly with (i) the time of reaction up to 400 min (r2 > 0.95) and (ii) α-pinene oxide gas-phase concentration. However, OS formation from α-pinene oxide reactions with slightly acidic or pure ammonium sulfate particles was limited, with a plateau ([OS]max = 0.62 ± 0.03 μg) reached after around 15-20 min. Organosulfate dimers (m/z 401 and m/z 481) were detected not only with highly acidic particles but also with slightly acidic and pure ammonium sulfate particles, indicating that oligomerization processes do not require strong acidity conditions. Dehydration products of organosulfates (m/z 231 and m/z 383) were observed only under highly acidic conditions, indicating the key role of H2SO4 on the dehydration of organosulfates and the formation of olefins in the atmosphere. Finally, this kinetic study was completed with simulation chamber experiments in which the mass concentration of organosulfates was shown to depend on the available sulfate amount present in the particle phase (r2 = 0.96). In conclusion, this relative comparison between five organosulfate precursors shows that epoxide was the most efficient reactant to form organosulfates via heterogeneous gas-particle reactions and illustrates how gas-particle reactions may play an important role in OS formation and hence in the atmospheric fate of organic carbon. The kinetic data presented in this work provide strong support to organosulfate formation mechanisms proposed in part 1 ( J. Phys. Chem. A 2016 , 120 , 7909 - 7923 ).
中文翻译:

α-P烯氧化形成有机硫酸盐的实验研究。2.时间演变和颗粒酸度的影响。
目前的工作是在三种不同的酸度条件下((NH4)2SO4 0.06 M;(NH4)2SO4 / H2SO4 0.06)中α-pine烯氧化产物或代理与酸化的硫酸铵气溶胶反应形成有机硫酸盐(OS)的广泛实验室研究。 M / 0.005 M;(NH 4)2 SO 4 / H 2 SO 4 0.03 M / 0.05 M)。使用准静态反应器研究了α-pine烯,α-pine烯氧化物,异op樟醇,pin二醇和myrtenal与硫酸铵颗粒反应的动力学。确定OS前体浓度为1时,α-pine烯氧化物与高酸性硫酸铵颗粒的反应分别比异海马酚,α-pine烯,松二烯和肉豆蔻的反应快7倍,10倍,21倍和24倍。 ppm和1小时反应时间后。确定在高酸性条件下由α-pine烯氧化物形成OS的有效速率系数比其他两种酸性条件高2个数量级。对于具有高酸性硫酸铵颗粒的α-pine烯氧化物反应,观察到OS形成随着(i)直至400分钟(r2> 0.95)的反应时间和(ii)α-pine烯氧化物气相浓度的增加而线性增加。然而,由α-pine烯氧化物与微酸性或纯硫酸铵颗粒反应形成的OS受到限制,约15-20分钟后达到平稳期([OS] max = 0.62±0.03μg)。不仅在高酸性颗粒中,而且在弱酸性和纯硫酸铵颗粒中都检测到了有机硫酸盐二聚体(m / z 401和m / z 481),表明低聚过程不需要强酸度条件。仅在高酸性条件下观察到有机硫酸盐的脱水产物(m / z 231和m / z 383),表明H2SO4在有机硫酸盐脱水和烯烃在大气中形成的关键作用。最后,通过模拟室实验完成了该动力学研究,其中有机硫酸盐的质量浓度显示取决于颗粒相中存在的可用硫酸盐量(r2 = 0.96)。综上所述,五个有机硫酸盐前体之间的这种相对比较表明,环氧化物是通过异质气体颗粒反应形成有机硫酸盐的最有效反应物,并说明了气体颗粒反应如何在OS形成以及有机碳的大气命运中起重要作用。这项工作中提供的动力学数据为第1部分中提出的有机硫酸盐形成机理提供了有力的支持(J. Phys。Chem。A 2016,120,7909-7923)。
更新日期:2020-01-04
中文翻译:

α-P烯氧化形成有机硫酸盐的实验研究。2.时间演变和颗粒酸度的影响。
目前的工作是在三种不同的酸度条件下((NH4)2SO4 0.06 M;(NH4)2SO4 / H2SO4 0.06)中α-pine烯氧化产物或代理与酸化的硫酸铵气溶胶反应形成有机硫酸盐(OS)的广泛实验室研究。 M / 0.005 M;(NH 4)2 SO 4 / H 2 SO 4 0.03 M / 0.05 M)。使用准静态反应器研究了α-pine烯,α-pine烯氧化物,异op樟醇,pin二醇和myrtenal与硫酸铵颗粒反应的动力学。确定OS前体浓度为1时,α-pine烯氧化物与高酸性硫酸铵颗粒的反应分别比异海马酚,α-pine烯,松二烯和肉豆蔻的反应快7倍,10倍,21倍和24倍。 ppm和1小时反应时间后。确定在高酸性条件下由α-pine烯氧化物形成OS的有效速率系数比其他两种酸性条件高2个数量级。对于具有高酸性硫酸铵颗粒的α-pine烯氧化物反应,观察到OS形成随着(i)直至400分钟(r2> 0.95)的反应时间和(ii)α-pine烯氧化物气相浓度的增加而线性增加。然而,由α-pine烯氧化物与微酸性或纯硫酸铵颗粒反应形成的OS受到限制,约15-20分钟后达到平稳期([OS] max = 0.62±0.03μg)。不仅在高酸性颗粒中,而且在弱酸性和纯硫酸铵颗粒中都检测到了有机硫酸盐二聚体(m / z 401和m / z 481),表明低聚过程不需要强酸度条件。仅在高酸性条件下观察到有机硫酸盐的脱水产物(m / z 231和m / z 383),表明H2SO4在有机硫酸盐脱水和烯烃在大气中形成的关键作用。最后,通过模拟室实验完成了该动力学研究,其中有机硫酸盐的质量浓度显示取决于颗粒相中存在的可用硫酸盐量(r2 = 0.96)。综上所述,五个有机硫酸盐前体之间的这种相对比较表明,环氧化物是通过异质气体颗粒反应形成有机硫酸盐的最有效反应物,并说明了气体颗粒反应如何在OS形成以及有机碳的大气命运中起重要作用。这项工作中提供的动力学数据为第1部分中提出的有机硫酸盐形成机理提供了有力的支持(J. Phys。Chem。A 2016,120,7909-7923)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号