当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prolonged quiescence delays somatic stem cell-like divisions in Caenorhabditis elegans and is controlled by insulin signaling.
Aging Cell ( IF 8.0 ) Pub Date : 2019-12-18 , DOI: 10.1111/acel.13085 María Olmedo 1 , Alejandro Mata-Cabana 1 , M J Rodríguez-Palero 2, 3 , Sabas García-Sánchez 1 , Antonio Fernández-Yañez 2, 3 , Martha Merrow 4 , Marta Artal-Sanz 2, 3
Aging Cell ( IF 8.0 ) Pub Date : 2019-12-18 , DOI: 10.1111/acel.13085 María Olmedo 1 , Alejandro Mata-Cabana 1 , M J Rodríguez-Palero 2, 3 , Sabas García-Sánchez 1 , Antonio Fernández-Yañez 2, 3 , Martha Merrow 4 , Marta Artal-Sanz 2, 3
Affiliation
|
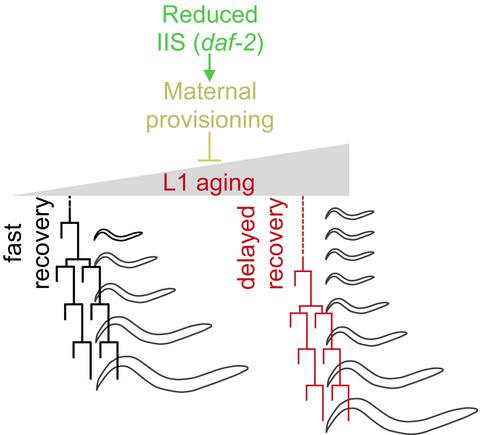
Cells can enter quiescence in adverse conditions and resume proliferation when the environment becomes favorable. Prolonged quiescence comes with a cost, reducing the subsequent speed and potential to return to proliferation. Here, we show that a similar process happens during Caenorhabditis elegans development, providing an in vivo model to study proliferative capacity after quiescence. Hatching under starvation provokes the arrest of blast cell divisions that normally take place during the first larval stage (L1). We have used a novel method to precisely quantify each stage of postembryonic development to analyze the consequences of prolonged L1 quiescence. We report that prolonged L1 quiescence delays the reactivation of blast cell divisions in C. elegans, leading to a delay in the initiation of postembryonic development. The transcription factor DAF‐16/FOXO is necessary for rapid recovery after extended arrest, and this effect is independent from its role as a suppressor of cell proliferation. Instead, the activation of DAF‐16 by decreased insulin signaling reduces the rate of L1 aging, increasing proliferative potential. We also show that yolk provisioning affects the proliferative potential after L1 arrest modulating the rate of L1 aging, providing a possible mechanistic link between insulin signaling and the maintenance of proliferative potential. Furthermore, variable yolk provisioning in embryos is one of the sources of interindividual variability in recovery after quiescence of genetically identical animals. Our results support the relevance of L1 arrest as an in vivo model to study stem cell‐like aging and the mechanisms for maintenance of proliferation potential after quiescence.
中文翻译:

长时间的静止会延迟秀丽隐杆线虫的体细胞干细胞样分裂,并受到胰岛素信号的控制。
细胞可以在不利条件下进入静止状态,并在环境变得有利时恢复增殖。长时间的静止是有代价的,会降低随后的速度和恢复扩散的可能性。在这里,我们证明了秀丽隐杆线虫发育过程中发生了类似的过程,为研究静止后的增殖能力提供了体内模型。饥饿条件下的孵化会引起母细胞分裂的停滞,而这种分裂通常发生在幼虫的第一个阶段(L1)。我们使用了一种新方法来精确量化胚胎后发育的每个阶段,以分析长期 L1 静止的后果。我们报告说,L1 长时间的静止会延迟线虫中母细胞分裂的重新激活,从而导致胚胎后发育启动的延迟。转录因子 DAF-16/FOXO 对于长时间停滞后的快速恢复是必需的,并且这种作用与其作为细胞增殖抑制剂的作用无关。相反,通过减少胰岛素信号激活 DAF-16 可降低 L1 衰老速度,增加增殖潜力。我们还表明,卵黄供应会影响 L1 停滞后的增殖潜力,从而调节 L1 老化速率,从而提供胰岛素信号传导与增殖潜力维持之间可能的机制联系。此外,胚胎中卵黄供应的变化是基因相同的动物静止后恢复过程中个体间差异的根源之一。我们的结果支持 L1 停滞作为体内模型研究干细胞样衰老和静止后维持增殖潜力的机制的相关性。
更新日期:2019-12-18
中文翻译:

长时间的静止会延迟秀丽隐杆线虫的体细胞干细胞样分裂,并受到胰岛素信号的控制。
细胞可以在不利条件下进入静止状态,并在环境变得有利时恢复增殖。长时间的静止是有代价的,会降低随后的速度和恢复扩散的可能性。在这里,我们证明了秀丽隐杆线虫发育过程中发生了类似的过程,为研究静止后的增殖能力提供了体内模型。饥饿条件下的孵化会引起母细胞分裂的停滞,而这种分裂通常发生在幼虫的第一个阶段(L1)。我们使用了一种新方法来精确量化胚胎后发育的每个阶段,以分析长期 L1 静止的后果。我们报告说,L1 长时间的静止会延迟线虫中母细胞分裂的重新激活,从而导致胚胎后发育启动的延迟。转录因子 DAF-16/FOXO 对于长时间停滞后的快速恢复是必需的,并且这种作用与其作为细胞增殖抑制剂的作用无关。相反,通过减少胰岛素信号激活 DAF-16 可降低 L1 衰老速度,增加增殖潜力。我们还表明,卵黄供应会影响 L1 停滞后的增殖潜力,从而调节 L1 老化速率,从而提供胰岛素信号传导与增殖潜力维持之间可能的机制联系。此外,胚胎中卵黄供应的变化是基因相同的动物静止后恢复过程中个体间差异的根源之一。我们的结果支持 L1 停滞作为体内模型研究干细胞样衰老和静止后维持增殖潜力的机制的相关性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号