当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubilities of Ammonia in Polyethylene Glycols at 298.2–353.2 K and 0–200 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-18 , DOI: 10.1021/acs.jced.9b00779 Jian-Bo Zhang 1 , Kuan Huang 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-12-18 , DOI: 10.1021/acs.jced.9b00779 Jian-Bo Zhang 1 , Kuan Huang 1
Affiliation
|
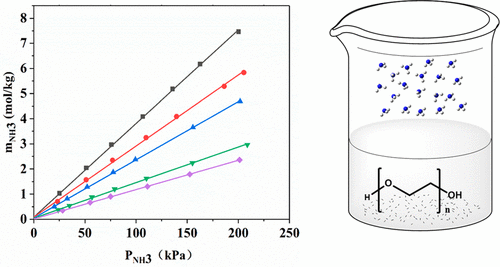
Polyethylene glycols (PEGs) are potential solvents for NH3 capture, considering that they have abundant hydroxyl groups, which can form hydrogen-bond interaction with NH3. To this end, the solubilities of NH3 in PEGs with average molecular weights of 100–600 g·mol–1 were measured at 298.2–353.2 K and 0–200 kPa in the present work. It is found that the solubilities of NH3 increase linearly with the increase of pressures, but decrease with the increase of temperatures. In addition, the solubilities of NH3 show negative dependence on the molecular weights of PEGs, because PEGs with higher molecular weights exhibit lower hydroxyl densities. The Henry’s constants and enthalpy changes for NH3 absorption in PEGs were also calculated based on the variations of NH3 solubilities with pressures and temperatures. Furthermore, the physical properties of PEGs that are essential for NH3 absorption such as densities and surface tensions were also measured at 293.2–353.2 K in the present work.
中文翻译:

氨在298.2–353.2 K和0–200 kPa时在聚乙二醇中的溶解度
考虑到聚乙二醇(PEG)具有丰富的羟基,可以与NH 3形成氢键相互作用,因此它们是捕获NH 3的潜在溶剂。为此,在目前的工作中,测得平均分子量为100–600 g·mol –1的PEG中NH 3的溶解度为298.2–353.2 K和0–200 kPa。发现NH 3的溶解度随着压力的增加而线性增加,但是随着温度的增加而减少。此外,NH 3的溶解度由于分子量较高的PEG的羟基密度较低,因此对PEG的分子量显示出负相关性。还基于NH 3溶解度随压力和温度的变化,计算了PEG中NH 3吸收的亨利常数和焓变。此外,在目前的工作中,也测量了对NH 3吸收至关重要的PEG的物理性质,例如密度和表面张力,其密度为293.2–353.2K。
更新日期:2019-12-19
中文翻译:

氨在298.2–353.2 K和0–200 kPa时在聚乙二醇中的溶解度
考虑到聚乙二醇(PEG)具有丰富的羟基,可以与NH 3形成氢键相互作用,因此它们是捕获NH 3的潜在溶剂。为此,在目前的工作中,测得平均分子量为100–600 g·mol –1的PEG中NH 3的溶解度为298.2–353.2 K和0–200 kPa。发现NH 3的溶解度随着压力的增加而线性增加,但是随着温度的增加而减少。此外,NH 3的溶解度由于分子量较高的PEG的羟基密度较低,因此对PEG的分子量显示出负相关性。还基于NH 3溶解度随压力和温度的变化,计算了PEG中NH 3吸收的亨利常数和焓变。此外,在目前的工作中,也测量了对NH 3吸收至关重要的PEG的物理性质,例如密度和表面张力,其密度为293.2–353.2K。











































 京公网安备 11010802027423号
京公网安备 11010802027423号