当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioisosteric Replacement of Amide Group with 1,2,3-Triazoles in Acetaminophen Addresses Reactive Oxygen Species-Mediated Hepatotoxic Insult in Wistar Albino Rats.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.chemrestox.9b00392 Adarsh Sahu 1 , Debashree Das 1 , Preeti Sahu 2 , Shweta Mishra 1 , Ayyamperumal Sakthivel 2 , Asmita Gajbhiye 1 , Ramkishore Agrawal 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-01-03 , DOI: 10.1021/acs.chemrestox.9b00392 Adarsh Sahu 1 , Debashree Das 1 , Preeti Sahu 2 , Shweta Mishra 1 , Ayyamperumal Sakthivel 2 , Asmita Gajbhiye 1 , Ramkishore Agrawal 1
Affiliation
|
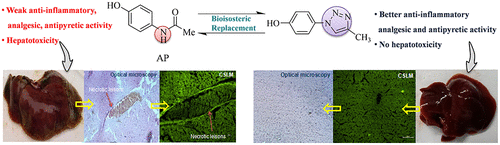
Acetaminophen (AP) is a popularly recommended over-the-counter analgesic-antipyretic in clinical use. However, the drug is handicapped by the occurrence of hepatotoxic insult following acute ingestion. Consequently, AP-induced hepatotoxicity is often implicated in accidental or suicidal overdose. In the current study, we investigated the potential of bioisosteric replacement of amide in AP with 1,2,3-triazoles in curbing AP-induced hepatotoxicity. The therapeutic utility of synthesized bioisosteres was established by careful tailoring and optimization of the synthetic methodology along with detailed toxicological testing of pharmacologically potent acetaminophen-triazole derivatives (APTDs). Along the same lines, we herein report a series of 17 novel APTDs synthesized via aromatic substitution using sodium azide, l-proline, and copper iodide followed by click reaction with substituted alkynes using copper sulfate and sodium ascorbate. Pharmacological evaluation of synthesized APTDs revealed that, out of the series of 17 compounds, 5a and 5e were found to be most efficacious in exerting anti-inflammatory, analgesic, and antipyretic activity in an animal model. Further toxicity studies documented that, in both acute and sub-acute toxicology, AP administration caused significant hepatotoxicity, which was found to be a consequence of ROS-mediated oxidative stress. Potent APTDs (5a and 5e), on the other hand, revealed no adverse event in both acute and sub-toxicological analyses. Median lethal dose (LD50) and no observed adverse effect level (NOAEL) values for 5a and 5e were found to be >1000 mg/kg and 2000 mg/kg, respectively. The human equivalent dose, defining the maximum safe concentration of a compound in a human's physiology, was found to be 27.68 mg/kg for the most potent APTDs (5a and 5e). Thus, it can be concluded that triazole incorporation into AP nucleus produced conjugates devoid of hepatotoxic manifestations, having the added advantage of anti-inflammatory efficacy along with analgesic and antipyretic potency.
中文翻译:

对乙酰氨基酚中的1,2,3-三唑类酰胺的生物体等位取代解决了Wistar白化病大鼠中活性氧介导的肝毒性损伤。
对乙酰氨基酚(AP)是临床上广泛推荐的非处方止痛解热药。但是,该药物因急性摄入后发生肝毒性损害而受到阻碍。因此,AP诱导的肝毒性通常与意外或自杀性过量有关。在当前的研究中,我们研究了用1,2,3-三唑类化合物在AP中进行生物等位取代酰胺以遏制AP诱导的肝毒性。合成生物等排体的治疗作用是通过仔细调整和优化合成方法,以及对药理学有效的对乙酰氨基酚-三唑衍生物(APTD)进行详细的毒理学测试而建立的。同样,我们在此报告了一系列使用叠氮化钠,l-脯氨酸通过芳族取代合成的17种新颖APTD,碘化铜,然后用硫酸铜和抗坏血酸钠与取代的炔烃进行点击反应。合成APTD的药理评估显示,在这17种化合物中,发现5a和5e在动物模型中发挥抗炎,止痛和解热活性最有效。进一步的毒性研究表明,在急性和亚急性毒理学中,AP给药均引起明显的肝毒性,这被认为是ROS介导的氧化应激的结果。另一方面,有效的APTD(5a和5e)在急性和亚毒理学分析中均未显示不良事件。发现5a和5e的中位致死剂量(LD50)和未观察到的不良影响水平(NOAEL)值分别> 1000 mg / kg和2000 mg / kg。人体等效剂量 对于最有效的APTD(5a和5e),确定化合物在人体生理学中的最大安全浓度的定义为27.68 mg / kg。因此,可以得出结论,将三唑掺入AP核中产生了没有肝毒性表现的结合物,具有抗炎功效以及止痛和解热效力的额外优势。
更新日期:2020-01-04
中文翻译:

对乙酰氨基酚中的1,2,3-三唑类酰胺的生物体等位取代解决了Wistar白化病大鼠中活性氧介导的肝毒性损伤。
对乙酰氨基酚(AP)是临床上广泛推荐的非处方止痛解热药。但是,该药物因急性摄入后发生肝毒性损害而受到阻碍。因此,AP诱导的肝毒性通常与意外或自杀性过量有关。在当前的研究中,我们研究了用1,2,3-三唑类化合物在AP中进行生物等位取代酰胺以遏制AP诱导的肝毒性。合成生物等排体的治疗作用是通过仔细调整和优化合成方法,以及对药理学有效的对乙酰氨基酚-三唑衍生物(APTD)进行详细的毒理学测试而建立的。同样,我们在此报告了一系列使用叠氮化钠,l-脯氨酸通过芳族取代合成的17种新颖APTD,碘化铜,然后用硫酸铜和抗坏血酸钠与取代的炔烃进行点击反应。合成APTD的药理评估显示,在这17种化合物中,发现5a和5e在动物模型中发挥抗炎,止痛和解热活性最有效。进一步的毒性研究表明,在急性和亚急性毒理学中,AP给药均引起明显的肝毒性,这被认为是ROS介导的氧化应激的结果。另一方面,有效的APTD(5a和5e)在急性和亚毒理学分析中均未显示不良事件。发现5a和5e的中位致死剂量(LD50)和未观察到的不良影响水平(NOAEL)值分别> 1000 mg / kg和2000 mg / kg。人体等效剂量 对于最有效的APTD(5a和5e),确定化合物在人体生理学中的最大安全浓度的定义为27.68 mg / kg。因此,可以得出结论,将三唑掺入AP核中产生了没有肝毒性表现的结合物,具有抗炎功效以及止痛和解热效力的额外优势。









































 京公网安备 11010802027423号
京公网安备 11010802027423号