当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction Energetics and Druggability of the Protein-Protein Interaction between Kelch-like ECH-Associated Protein 1 (KEAP1) and Nuclear Factor Erythroid 2 Like 2 (Nrf2).
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.biochem.9b00943 Mengqi Zhong , Andrew Lynch , Samantha N Muellers , Stefan Jehle , Lingqi Luo , David R Hall 1 , Reina Iwase , James P Carolan , Megan Egbert , Amanda Wakefield , Kristina Streu , Christine M Harvey , Paula C Ortet , Dima Kozakov 2 , Sandor Vajda 3 , Karen N Allen 3 , Adrian Whitty 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.biochem.9b00943 Mengqi Zhong , Andrew Lynch , Samantha N Muellers , Stefan Jehle , Lingqi Luo , David R Hall 1 , Reina Iwase , James P Carolan , Megan Egbert , Amanda Wakefield , Kristina Streu , Christine M Harvey , Paula C Ortet , Dima Kozakov 2 , Sandor Vajda 3 , Karen N Allen 3 , Adrian Whitty 3
Affiliation
|
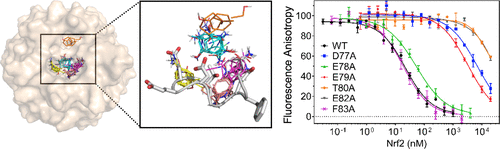
Development of small molecule inhibitors of protein-protein interactions (PPIs) is hampered by our poor understanding of the druggability of PPI target sites. Here, we describe the combined application of alanine-scanning mutagenesis, fragment screening, and FTMap computational hot spot mapping to evaluate the energetics and druggability of the highly charged PPI interface between Kelch-like ECH-associated protein 1 (KEAP1) and nuclear factor erythroid 2 like 2 (Nrf2), an important drug target. FTMap identifies four binding energy hot spots at the active site. Only two of these are exploited by Nrf2, which alanine scanning of both proteins shows to bind primarily through E79 and E82 interacting with KEAP1 residues S363, R380, R415, R483, and S508. We identify fragment hits and obtain X-ray complex structures for three fragments via crystal soaking using a new crystal form of KEAP1. Combining these results provides a comprehensive and quantitative picture of the origins of binding energy at the interface. Our findings additionally reveal non-native interactions that might be exploited in the design of uncharged synthetic ligands to occupy the same site on KEAP1 that has evolved to bind the highly charged DEETGE binding loop of Nrf2. These include π-stacking with KEAP1 Y525 and interactions at an FTMap-identified hot spot deep in the binding site. Finally, we discuss how the complementary information provided by alanine-scanning mutagenesis, fragment screening, and computational hot spot mapping can be integrated to more comprehensively evaluate PPI druggability.
中文翻译:

Kelch 样 ECH 相关蛋白 1 (KEAP1) 和核因子红细胞 2 样 2 (Nrf2) 之间蛋白质-蛋白质相互作用的相互作用能量学和成药性。
由于我们对 PPI 靶位点的成药性了解不足,阻碍了蛋白质-蛋白质相互作用 (PPI) 小分子抑制剂的开发。在这里,我们描述了丙氨酸扫描诱变、片段筛选和 FTMap 计算热点映射的组合应用,以评估 Kelch 样 ECH 相关蛋白 1 (KEAP1) 和核因子红细胞之间的高电荷 PPI 界面的能量学和成药性2 like 2 (Nrf2),一个重要的药物靶点。FTMap 识别出活性位点的四个结合能热点。其中只有两个被 Nrf2 利用,对两种蛋白的丙氨酸扫描显示,Nrf2 主要通过 E79 和 E82 与 KEAP1 残基 S363、R380、R415、R483 和 S508 相互作用进行结合。我们使用 KEAP1 的新晶型通过晶体浸泡来识别碎片命中并获得了三个碎片的 X 射线复合结构。结合这些结果提供了界面结合能起源的全面、定量的图像。我们的研究结果还揭示了非天然相互作用,可用于设计不带电荷的合成配体,以占据 KEAP1 上的同一位点,该位点已进化为结合 Nrf2 的高电荷 DEETGE 结合环。其中包括与 KEAP1 Y525 的 π 堆积以及在结合位点深处 FTMap 识别的热点处的相互作用。最后,我们讨论了如何整合丙氨酸扫描诱变、片段筛选和计算热点图谱提供的补充信息,以更全面地评估 PPI 成药性。
更新日期:2020-01-04
中文翻译:

Kelch 样 ECH 相关蛋白 1 (KEAP1) 和核因子红细胞 2 样 2 (Nrf2) 之间蛋白质-蛋白质相互作用的相互作用能量学和成药性。
由于我们对 PPI 靶位点的成药性了解不足,阻碍了蛋白质-蛋白质相互作用 (PPI) 小分子抑制剂的开发。在这里,我们描述了丙氨酸扫描诱变、片段筛选和 FTMap 计算热点映射的组合应用,以评估 Kelch 样 ECH 相关蛋白 1 (KEAP1) 和核因子红细胞之间的高电荷 PPI 界面的能量学和成药性2 like 2 (Nrf2),一个重要的药物靶点。FTMap 识别出活性位点的四个结合能热点。其中只有两个被 Nrf2 利用,对两种蛋白的丙氨酸扫描显示,Nrf2 主要通过 E79 和 E82 与 KEAP1 残基 S363、R380、R415、R483 和 S508 相互作用进行结合。我们使用 KEAP1 的新晶型通过晶体浸泡来识别碎片命中并获得了三个碎片的 X 射线复合结构。结合这些结果提供了界面结合能起源的全面、定量的图像。我们的研究结果还揭示了非天然相互作用,可用于设计不带电荷的合成配体,以占据 KEAP1 上的同一位点,该位点已进化为结合 Nrf2 的高电荷 DEETGE 结合环。其中包括与 KEAP1 Y525 的 π 堆积以及在结合位点深处 FTMap 识别的热点处的相互作用。最后,我们讨论了如何整合丙氨酸扫描诱变、片段筛选和计算热点图谱提供的补充信息,以更全面地评估 PPI 成药性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号