Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
β-Arrestin-2 BRET Biosensors Detect Different β-Arrestin-2 Conformations in Interaction with GPCRs.
ACS Sensors ( IF 8.9 ) Pub Date : 2019-12-31 , DOI: 10.1021/acssensors.9b01414 Atsuro Oishi 1 , Julie Dam 1 , Ralf Jockers 1
ACS Sensors ( IF 8.9 ) Pub Date : 2019-12-31 , DOI: 10.1021/acssensors.9b01414 Atsuro Oishi 1 , Julie Dam 1 , Ralf Jockers 1
Affiliation
|
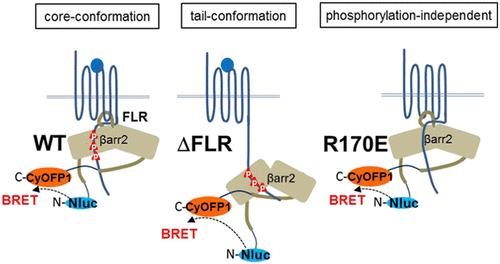
β-Arrestins are critical regulators of G protein-coupled receptors (GPCRs) that desensitize G protein signaling, promote receptor internalization, and initiate signaling on their own. Recent structural findings indicate that β-arrestins adopt different conformations upon interaction with agonist-activated GPCRs. Here, we established a β-arrestin-2 conformational bioluminescence resonance energy transfer (BRET) sensor composed of the bright Nanoluc BRET donor and the red-shifted CyOFP1 BRET acceptor. The sensor monitors early intramolecular conformational changes of β-arrestin-2 in complex with a wide panel of different class A and class B GPCRs upon agonist activation and with orphan GPCRs known to spontaneously recruit β-arrestin-2. The introduction of the R170E mutant in the β-arrestin-2 sensor allowed the detection of a partially active β-arrestin-2 conformation, which is not dependent on receptor phosphorylation, while the deletion of the β-arrestin-2 finger-loop region detected the "tail-conformation" corresponding to the interaction of β-arrestin with the carboxyl-terminal domain of GPCRs. The new sensors combine the advantages of the BRET technique in terms of sensitivity, robustness, and suitability for real-time measurements with a high responsiveness toward early conformational changes to help to elucidate the different conformational states of β-arrestins associated with GPCR activation in living cells.
中文翻译:

β-Arrestin-2BRET生物传感器可与GPCR相互作用检测不同的β-Arrestin-2构象。
β-Arrestins是G蛋白偶联受体(GPCR)的关键调节剂,可以使G蛋白信号转敏,促进受体内化并自行启动信号。最近的结构发现表明,β-arrestin在与激动剂激活的GPCR相互作用时采用不同的构象。在这里,我们建立了由明亮的Nanoluc BRET供体和红移CyOFP1 BRET受体组成的β-arrestin-2构象生物发光共振能量转移(BRET)传感器。该传感器可通过激动剂激活后与大量不同的A类和B类GPCR以及已知自发募集β-arrestin-2的孤儿GPCR一起监测复合物中β-arrestin-2的早期分子内构象变化。将R170E突变体引入β-arrestin-2传感器后,可以检测到部分活性的β-arrestin-2构象,该构象不依赖于受体的磷酸化,而缺失了β-arrestin-2的指环区。检测到与β-arrestin与GPCR的羧基末端结构域的相互作用相对应的“尾巴构象”。新型传感器结合了BRET技术的优势,在灵敏度,鲁棒性和适用于实时测量的方面,以及对早期构象变化的高响应性,有助于阐明与GPCR激活相关的β-arrestin的不同构象状态。细胞。
更新日期:2020-01-01
中文翻译:

β-Arrestin-2BRET生物传感器可与GPCR相互作用检测不同的β-Arrestin-2构象。
β-Arrestins是G蛋白偶联受体(GPCR)的关键调节剂,可以使G蛋白信号转敏,促进受体内化并自行启动信号。最近的结构发现表明,β-arrestin在与激动剂激活的GPCR相互作用时采用不同的构象。在这里,我们建立了由明亮的Nanoluc BRET供体和红移CyOFP1 BRET受体组成的β-arrestin-2构象生物发光共振能量转移(BRET)传感器。该传感器可通过激动剂激活后与大量不同的A类和B类GPCR以及已知自发募集β-arrestin-2的孤儿GPCR一起监测复合物中β-arrestin-2的早期分子内构象变化。将R170E突变体引入β-arrestin-2传感器后,可以检测到部分活性的β-arrestin-2构象,该构象不依赖于受体的磷酸化,而缺失了β-arrestin-2的指环区。检测到与β-arrestin与GPCR的羧基末端结构域的相互作用相对应的“尾巴构象”。新型传感器结合了BRET技术的优势,在灵敏度,鲁棒性和适用于实时测量的方面,以及对早期构象变化的高响应性,有助于阐明与GPCR激活相关的β-arrestin的不同构象状态。细胞。



























 京公网安备 11010802027423号
京公网安备 11010802027423号