当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption of Methyl Chloride on Molecular Sieves, Silica Gels, and Activated Carbon
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-01-21 , DOI: 10.1002/ceat.201900199 Jai B. Lad 1 , Yassir T. Makkawi 2
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2020-01-21 , DOI: 10.1002/ceat.201900199 Jai B. Lad 1 , Yassir T. Makkawi 2
Affiliation
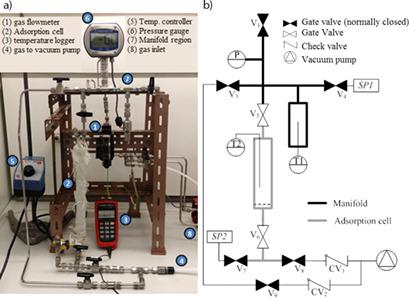
|
Adsorption of methyl chloride (CH3Cl or MeCl) on five different types of adsorbents was investigated experimentally at increasing pressures and room temperature. Prior to adsorption, all adsorbents were analyzed to assess their physical and chemical characteristics. The experimental data was then used to determine the adsorption isotherms, heats of adsorption, adsorption rates, and their respective theoretical models. The MeCl adsorption capacity was found to reasonably correlate with the adsorbent's surface area. The MeCl adsorption isotherm and adsorption rates were fitted for the first time to a Freundlich isotherm model and pseudo first‐/second‐order kinetic models, respectively. The range of heat of adsorption indicated a physisorption type of bonding; hence, the investigated adsorbents can potentially be regenerated for cyclic adsorption.
中文翻译:

氯甲烷在分子筛,硅胶和活性炭上的吸附
吸附氯甲烷(CH 3在增加的压力和室温下,通过实验研究了五种不同类型吸附剂上的Cl或MeCl)。吸附之前,对所有吸附剂进行分析以评估其物理和化学特性。然后将实验数据用于确定吸附等温线,吸附热,吸附速率及其各自的理论模型。发现MeCl的吸附容量与吸附剂的表面积合理地相关。MeCl吸附等温线和吸附速率首次分别拟合到Freundlich等温线模型和伪一级/二级动力学模型。吸附热的范围表明了键的物理吸附类型。因此,所研究的吸附剂可以潜在地再生用于循环吸附。
更新日期:2020-01-21
中文翻译:

氯甲烷在分子筛,硅胶和活性炭上的吸附
吸附氯甲烷(CH 3在增加的压力和室温下,通过实验研究了五种不同类型吸附剂上的Cl或MeCl)。吸附之前,对所有吸附剂进行分析以评估其物理和化学特性。然后将实验数据用于确定吸附等温线,吸附热,吸附速率及其各自的理论模型。发现MeCl的吸附容量与吸附剂的表面积合理地相关。MeCl吸附等温线和吸附速率首次分别拟合到Freundlich等温线模型和伪一级/二级动力学模型。吸附热的范围表明了键的物理吸附类型。因此,所研究的吸附剂可以潜在地再生用于循环吸附。











































 京公网安备 11010802027423号
京公网安备 11010802027423号