Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-18 , DOI: 10.1016/j.tet.2019.130885 Kukkamudi Sreenivas , Faiz Ahmed Khan
|
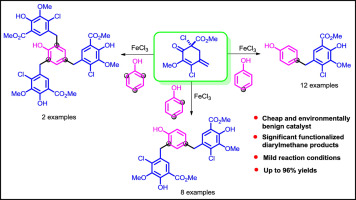
An efficient synthesis of diarylmethane derivatives from phenol and a p-quinone methide surrogate cyclohexadienone derivative has been described. This 1,6-conjugate addition reaction is catalyzed by earth abundant FeCl3 and it proceeds via a consecutive Michael type addition followed by aromatization. Various proportions of phenol and cyclohexadienone produced exclusively mono, bis and tris addition products. Interestingly, di-tert-butylphenol gave addition product with concomitant loss of one of the tertiary butyl groups of the phenol moiety via retro-Friedel-Crafts reaction. This strategy demonstrates a straightforward access to wide range of diarylmethane derivatives possessing biologically significant ortho-methoxyphenol moiety under mild reaction conditions.
中文翻译:

FeCl 3催化苯酚C-亲核试剂的1,6-共轭加成:轻松合成二芳基甲烷
已经描述了由苯酚和对苯醌甲基化物替代环己二酮衍生物有效地合成二芳基甲烷衍生物。该1,6-共轭加成反应由富含稀土的FeCl 3催化,并通过连续的迈克尔型加成随后进行芳构化而进行。各种比例的苯酚和环己二酮只生产单,双和三加成产物。有趣的是,二-叔丁基苯酚,得到加成产物经由复古Friedel-Crafts反应的苯酚部分的叔丁基基团之一的同时损失。该策略表明可以直接获得具有生物学上显着邻位性的各种二芳基甲烷衍生物-甲氧基苯酚部分在温和的反应条件下。











































 京公网安备 11010802027423号
京公网安备 11010802027423号