当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antiproliferative activities of tricyclic amides derived from β-caryophyllene via the Ritter reaction against MDA-MB-231 breast cancer cells
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019/12/18 , DOI: 10.1039/c9md00237e XiXi Xu 1 , Ariane Roseblade 1 , Tristan Rawling 1 , Alison T Ung 1
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2019/12/18 , DOI: 10.1039/c9md00237e XiXi Xu 1 , Ariane Roseblade 1 , Tristan Rawling 1 , Alison T Ung 1
Affiliation
|
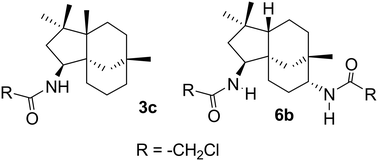
A library of novel tricyclic amides has been synthesised via the Ritter reaction from β-caryophyllene 1 and its monoepoxy derivative 4. The compounds were assessed for antiproliferative activities against the aggressive MDA-MB-231 breast cancer cell line. Of the synthesised compounds, eight were active. 3c and 6b were the most potent and inhibited proliferation with IC50 of 9.7 and 8.2 μM, respectively. Mechanistic studies revealed differences in their antiproliferative actions. 6b inhibited cell cycle progression and induced predominantly apoptotic cell death. In contrast, 3c did not affect cell cycle kinetics and favoured necrotic over apoptotic pathways. Screening against mammalian cells (VERO cells) indicates that 3c and 6b were more active towards MDA-MB-231 cells than noncancerous cells. Facile synthesis and biological results suggest that these caryophyllene derived amides are viable lead compounds for further development.
中文翻译:

β-石竹烯衍生的三环酰胺通过 Ritter 反应对 MDA-MB-231 乳腺癌细胞的抗增殖活性
通过Ritter 反应,由 β-石竹烯1及其单环氧衍生物4合成了一系列新型三环酰胺。评估了这些化合物对侵袭性 MDA-MB-231 乳腺癌细胞系的抗增殖活性。在合成的化合物中,有八种具有活性。 3c和6b是最有效且抑制增殖的,IC 50分别为 9.7 和 8.2 μM。机理研究揭示了它们的抗增殖作用的差异。 6b抑制细胞周期进程并主要诱导细胞凋亡。相比之下, 3c不影响细胞周期动力学,并且有利于坏死途径而不是凋亡途径。针对哺乳动物细胞(VERO 细胞)的筛选表明, 3c和6b对 MDA-MB-231 细胞比非癌细胞更具活性。简单的合成和生物学结果表明这些石竹烯衍生的酰胺是可用于进一步开发的可行的先导化合物。
更新日期:2020-02-13
中文翻译:

β-石竹烯衍生的三环酰胺通过 Ritter 反应对 MDA-MB-231 乳腺癌细胞的抗增殖活性
通过Ritter 反应,由 β-石竹烯1及其单环氧衍生物4合成了一系列新型三环酰胺。评估了这些化合物对侵袭性 MDA-MB-231 乳腺癌细胞系的抗增殖活性。在合成的化合物中,有八种具有活性。 3c和6b是最有效且抑制增殖的,IC 50分别为 9.7 和 8.2 μM。机理研究揭示了它们的抗增殖作用的差异。 6b抑制细胞周期进程并主要诱导细胞凋亡。相比之下, 3c不影响细胞周期动力学,并且有利于坏死途径而不是凋亡途径。针对哺乳动物细胞(VERO 细胞)的筛选表明, 3c和6b对 MDA-MB-231 细胞比非癌细胞更具活性。简单的合成和生物学结果表明这些石竹烯衍生的酰胺是可用于进一步开发的可行的先导化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号