当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Synthesis of Monoesters of Itaconic Acid with Broad Substrate Scope: Biobased Alternatives to Acrylic Acid?
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-01-13 , DOI: 10.1021/acssuschemeng.9b06330 Sacha Pérocheau Arnaud 1 , Eleni Andreou 1 , Luis V. G. Pereira Köster 1 , Tobias Robert 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-01-13 , DOI: 10.1021/acssuschemeng.9b06330 Sacha Pérocheau Arnaud 1 , Eleni Andreou 1 , Luis V. G. Pereira Köster 1 , Tobias Robert 1
Affiliation
|
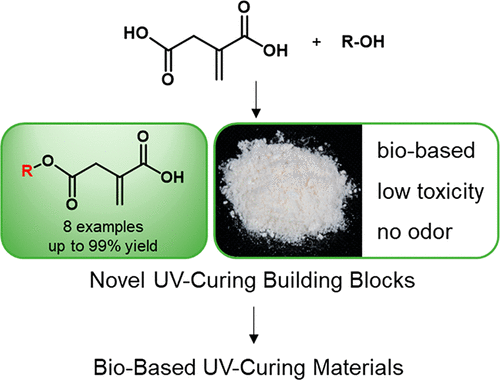
Over the last few years, there has been an increasing interest to identify biobased alternatives to acrylic acid in UV-curing materials. In this respect, itaconic acid has drawn considerable attention, due to its structural similarity. However, the second acid group in the molecule limits its use in many applications, as undesired side reactions can occur. One solution is the use of monoesters of itaconic acid to mimic the properties of acrylic acid. However, to date, no general applicable and straightforward synthetic protocol has been reported. Herein, the synthesis of a variety of monoesters of itaconic acid is presented. The methodology tolerates a broad substrate scope, is highly efficient, and does not suffer from tedious purification steps; therefore it can be applied on large scale. In the next step, the monoesters were reacted with epoxidized soybean oil to produce biobased UV-curing oligomers. The influence of the different monoesters on the properties of the functionalized vegetable oils, such as viscosity as well as the reactivity toward UV-light induced radical cross-linking, was studied. In addition, these findings were compared with those of acrylated soybean oil. Despite the lower rate of polymerization of the itaconic acid-based oligomers, the double bond conversion was higher in some cases. In addition, the viscosity was found to be lower in comparison to that of the acrylic acid-derived compound, making these novel monoesters promising alternatives to the petrochemical-based acrylic acid.
中文翻译:

具有广泛底物的衣康酸单酯的选择性合成范围:丙烯酸的生物替代品?
在过去的几年中,人们越来越关注在紫外线固化材料中鉴定丙烯酸的生物基替代品。在这方面,衣康酸由于其结构相似性而备受关注。但是,分子中的第二个酸基团会限制其在许多应用中的使用,因为会发生不希望的副反应。一种解决方案是使用衣康酸的单酯来模拟丙烯酸的性质。然而,迄今为止,尚未报道普遍适用且直接的合成方案。在此,提出了衣康酸的各种单酯的合成。该方法可耐受较宽的底物范围,效率高,且无需繁琐的纯化步骤;因此可以大规模应用。在下一步中 单酯与环氧化大豆油反应生成生物基紫外线固化低聚物。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。
更新日期:2020-01-14
中文翻译:

具有广泛底物的衣康酸单酯的选择性合成范围:丙烯酸的生物替代品?
在过去的几年中,人们越来越关注在紫外线固化材料中鉴定丙烯酸的生物基替代品。在这方面,衣康酸由于其结构相似性而备受关注。但是,分子中的第二个酸基团会限制其在许多应用中的使用,因为会发生不希望的副反应。一种解决方案是使用衣康酸的单酯来模拟丙烯酸的性质。然而,迄今为止,尚未报道普遍适用且直接的合成方案。在此,提出了衣康酸的各种单酯的合成。该方法可耐受较宽的底物范围,效率高,且无需繁琐的纯化步骤;因此可以大规模应用。在下一步中 单酯与环氧化大豆油反应生成生物基紫外线固化低聚物。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。研究了不同单酯对官能化植物油性能的影响,例如粘度以及对紫外线诱导的自由基交联的反应性。此外,将这些发现与丙烯酸大豆油进行了比较。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。尽管衣康酸基低聚物的聚合速率较低,但在某些情况下双键转化率较高。另外,发现其粘度低于丙烯酸衍生的化合物的粘度,这使得这些新颖的单酯有望成为石油化学基丙烯酸的替代品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号