当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetic Relaxation Studies on Trigonal Bipyramidal Cobalt(II) Complexes.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-01-16 , DOI: 10.1002/asia.201901511 Feng Shao 1, 2, 3 , Benjamin Cahier 1, 4 , Yi-Ting Wang 1, 2 , Feng-Lei Yang 1, 2, 5 , Eric Rivière 1 , Régis Guillot 1 , Nathalie Guihéry 6 , Jia-Ping Tong 2, 7 , Talal Mallah 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2020-01-16 , DOI: 10.1002/asia.201901511 Feng Shao 1, 2, 3 , Benjamin Cahier 1, 4 , Yi-Ting Wang 1, 2 , Feng-Lei Yang 1, 2, 5 , Eric Rivière 1 , Régis Guillot 1 , Nathalie Guihéry 6 , Jia-Ping Tong 2, 7 , Talal Mallah 1
Affiliation
|
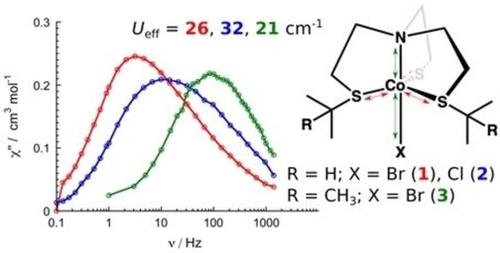
We report the preparation and the full characterization of a novel mononuclear trigonal bipyramidal CoII complex [Co(NS3 iPr )Br](BPh4 ) (1) with the tetradentate sulfur-containing ligand NS3 iPr (N(CH2 CH2 SCH(CH3 )2 )3 ). The comparison of its magnetic behaviour with those of two previously reported compounds [Co(NS3 iPr )Cl](BPh4 ) (2) and [Co(NS3 tBu )Br](ClO4 ) (3) (NS3 tBu =N(CH2 CH2 SC(CH3 )3 )3 ) with similar structures shows that 1 displays a single-molecule magnet behaviour with the longest magnetic relaxation time (0.051 s) at T=1.8 K, which is almost thirty times larger than that of 3 (0.0019 s) and more than three times larger than that of 2 (0.015 s), though its effective energy barrier (26 cm-1 ) is smaller. Compound 1, which contains two crystallographically independent molecules, presents smaller rhombic parameters (E=1.45 and 0.59 cm-1 ) than 2 (E=2.05 and 1.02 cm-1 ) and 3 (E=2.00 and 0.80 cm-1 ) obtained from theoretical calculations. Compounds 2 and 3 have almost the same axial (D) and rhombic (E) parameter values, but present a large difference of their effective energy barrier and magnetic relaxation which may be attributed to the larger volume of BPh4 - than ClO4 - leading to larger diamagnetic dilution (weaker magnetic dipolar interaction) for 2 than for 3. The combination of these factors leads to a much slower magnetic relaxation for 1 than for the two other compounds.
中文翻译:

三角双锥钴(II)配合物的磁弛豫研究。
我们报告的新型四核含硫配体NS3 iPr(N(CH2 CH2 SCH(CH3)2)的新型单核三角双锥双CoII复合物[Co(NS3 iPr)Br](BPh4)(1) 3)。与之前报道的两种化合物[Co(NS3 iPr)Cl](BPh4)(2)和[Co(NS3 tBu)Br](ClO4)(3)的磁性比较(NS3 tBu = N(CH2 CH2具有相似结构的SC(CH3)3)3)显示1在T = 1.8 K时显示的单分子磁体行为具有最长的磁弛豫时间(0.051 s),几乎是3(0.0019 s)的三十倍),但其有效能垒(26 cm-1)较小,但大于2的三倍(0.015 s)。化合物1包含两个晶体学独立的分子,具有较小的菱形参数(E = 1.45和0。从理论计算获得的2(E = 2.05和1.02 cm-1)和3(E = 2.00和0.80 cm-1)比59 cm-1)大。化合物2和3的轴向(D)和菱形(E)参数值几乎相同,但化合物的有效能垒和磁弛豫差异很大,这可能归因于BPh4的体积比ClO4的体积大,导致BPh4的体积更大抗磁性稀释(较弱的磁偶极相互作用)为2时比3稀释。这些因素的组合导致1的磁弛豫要比其他两种化合物慢得多。
更新日期:2020-01-16
中文翻译:

三角双锥钴(II)配合物的磁弛豫研究。
我们报告的新型四核含硫配体NS3 iPr(N(CH2 CH2 SCH(CH3)2)的新型单核三角双锥双CoII复合物[Co(NS3 iPr)Br](BPh4)(1) 3)。与之前报道的两种化合物[Co(NS3 iPr)Cl](BPh4)(2)和[Co(NS3 tBu)Br](ClO4)(3)的磁性比较(NS3 tBu = N(CH2 CH2具有相似结构的SC(CH3)3)3)显示1在T = 1.8 K时显示的单分子磁体行为具有最长的磁弛豫时间(0.051 s),几乎是3(0.0019 s)的三十倍),但其有效能垒(26 cm-1)较小,但大于2的三倍(0.015 s)。化合物1包含两个晶体学独立的分子,具有较小的菱形参数(E = 1.45和0。从理论计算获得的2(E = 2.05和1.02 cm-1)和3(E = 2.00和0.80 cm-1)比59 cm-1)大。化合物2和3的轴向(D)和菱形(E)参数值几乎相同,但化合物的有效能垒和磁弛豫差异很大,这可能归因于BPh4的体积比ClO4的体积大,导致BPh4的体积更大抗磁性稀释(较弱的磁偶极相互作用)为2时比3稀释。这些因素的组合导致1的磁弛豫要比其他两种化合物慢得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号