The Lancet Global Health ( IF 19.9 ) Pub Date : 2019-12-18 , DOI: 10.1016/s2214-109x(19)30541-8 Myron M Levine 1 , Dilruba Nasrin 2 , Sozinho Acácio 3 , Quique Bassat 4 , Helen Powell 5 , Sharon M Tennant 2 , Samba O Sow 6 , Dipika Sur 7 , Anita K M Zaidi 8 , Abu S G Faruque 9 , M Jahangir Hossain 10 , Pedro L Alonso 11 , Robert F Breiman 12 , Ciara E O'Reilly 13 , Eric D Mintz 14 , Richard Omore 15 , John B Ochieng 15 , Joseph O Oundo 16 , Boubou Tamboura 6 , Doh Sanogo 6 , Uma Onwuchekwa 6 , Byomkesh Manna 7 , Thandavarayan Ramamurthy 17 , Suman Kanungo 7 , Shahnawaz Ahmed 9 , Shahida Qureshi 18 , Farheen Quadri 19 , Anowar Hossain 20 , Sumon K Das 21 , Martin Antonio 10 , Debasish Saha 22 , Inacio Mandomando 3 , William C Blackwelder 23 , Tamer Farag 24 , Yukun Wu 25 , Eric R Houpt 26 , Jaco J Verweiij 27 , Halvor Sommerfelt 28 , James P Nataro 29 , Roy M Robins-Browne 30 , Karen L Kotloff 1
|
Background
The Global Enteric Multicenter Study (GEMS) was a 3-year case-control study that measured the burden, aetiology, and consequences of moderate-to-severe diarrhoea (MSD) in children aged 0–59 months. GEMS-1A, a 12-month follow-on study, comprised two parallel case-control studies, one assessing MSD and the other less-severe diarrhoea (LSD). In this report, we analyse the risk of death with each diarrhoea type and the specific pathogens associated with fatal outcomes.
Methods
GEMS was a prospective, age-stratified, matched case-control study done at seven sites in Africa and Asia. Children aged 0–59 months with MSD seeking care at sentinel health centres were recruited along with one to three randomly selected matched community control children without diarrhoea. In the 12-month GEMS-1A follow-on study, children with LSD and matched controls, in addition to children with MSD and matched controls, were recruited at six of the seven sites; only cases of MSD and controls were enrolled at the seventh site. We compared risk of death during the period between enrolment and one follow-up household visit done about 60 days later (range 50–90 days) in children with MSD and LSD and in their respective controls. Approximately 50 pathogens were detected using, as appropriate, classic bacteriology, immunoassays, gel-based PCR and reverse transcriptase PCR, and quantitative real-time PCR (qPCR). Specimens from a subset of GEMS cases and controls were also tested by a TaqMan Array Card that compartmentalised probe-based qPCR for 32 enteropathogens.
Findings
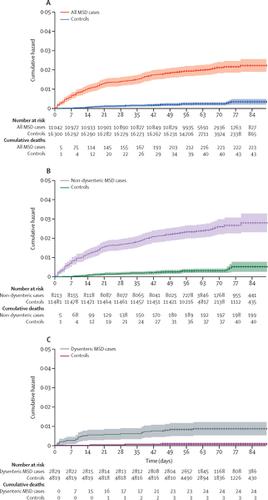
223 (2·0%) of 11 108 children with MSD and 43 (0·3%) of 16 369 matched controls died between study enrolment and the follow-up visit at about 60 days (hazard ratio [HR] 8·16, 95% CI 5·69–11·68, p<0·0001). 12 (0·4%) of 2962 children with LSD and seven (0·2%) of 4074 matched controls died during the follow-up period (HR 2·78, 95% CI 0·95–8·11, p=0·061). Risk of death was lower in children with dysenteric MSD than in children with non-dysenteric MSD (HR 0·20, 95% CI 0·05–0·87, p=0·032), and lower in children with LSD than in those with non-dysenteric MSD (HR 0·29, 0·14–0·59, p=0·0006). In children younger than 24 months with MSD, infection with typical enteropathogenic Escherichia coli, enterotoxigenic E coli encoding heat-stable toxin, enteroaggregative E coli, Shigella spp (non-dysentery cases), Aeromonas spp, Cryptosporidium spp, and Entamoeba histolytica increased risk of death. Of 61 deaths in children aged 12–59 months with non-dysenteric MSD, 31 occurred among 942 children qPCR-positive for Shigella spp and 30 deaths occurred in 1384 qPCR-negative children (HR 2·2, 95% CI 1·2–3·9, p=0·0090), showing that Shigella was strongly associated with increased risk of death.
Interpretation
Risk of death is increased following MSD and, to a lesser extent, LSD. Considering there are approximately three times more cases of LSD than MSD in the population, more deaths are expected among children with LSD than in those with MSD. Because the major attributable LSD-associated and MSD-associated pathogens are the same, implementing vaccines and rapid diagnosis and treatment interventions against these major pathogens are rational investments.
Funding
Bill & Melinda Gates Foundation.
中文翻译:

低收入和中等收入国家的婴幼儿腹泻病和随后的死亡风险:GEMS病例对照研究和12个月GEMS-1A后续研究的分析。
背景
全球肠道多中心研究(GEMS)是一项为期3年的病例对照研究,旨在测量0-59个月大的儿童的负担,病因和中度至重度腹泻(MSD)的后果。GEMS-1A是一项为期12个月的后续研究,包括两项平行的病例对照研究,一项评估MSD,另一项评估轻度腹泻(LSD)。在本报告中,我们分析了每种腹泻类型的死亡风险以及与致命结果相关的特定病原体。
方法
GEMS是一项在非洲和亚洲七个地区进行的前瞻性,年龄分层,匹配病例对照研究。招募了在前哨医疗中心寻求MSD治疗的0-59个月的MSD儿童,以及一到三名随机选择的匹配的无腹泻社区控制儿童。在为期12个月的GEMS-1A后续研究中,从7个地点中的6个招募了患有LSD和配对对照的儿童以及MSD和配对对照的儿童。在第7个站点仅登记了MSD和对照病例。我们比较了在MSD和LSD患儿及其各自对照组中,在入组与约60天后(50-90天不等)进行一次随访家庭访视之间的死亡风险。使用经典细菌学,免疫分析,基于凝胶的PCR和逆转录酶PCR,以及定量实时PCR(qPCR)。TaqMan阵列卡还测试了一部分GEMS病例和对照的标本,该卡将基于探针的qPCR分隔为32种肠病原体。
发现
在研究入组与随访之间约60天时,有11108名MSD儿童中的223名(2·0%)和16369名配对对照中的43名(0·3%)死亡(危险比[HR] 8·16, 95%CI 5·69-11·68,p <0·0001)。2962名LSD儿童中有12名(0·4%)和4074名配对对照中有7名(0·2%)在随访期间死亡(HR 2·78,95%CI 0·95-8·11,p = 0·061)。痢疾MSD患儿的死亡风险低于非痢疾MSD患儿(HR 0·20,95%CI 0·05-0·87,p = 0·032),而LSD患儿的死亡风险低于非DDS患儿。患有非痢疾性MSD的患者(HR 0·29,0·14–0·59,p = 0·0006)。在儿童年龄小于24个月,MSD,感染肠致病性典型埃希氏大肠杆菌,肠产毒性大肠杆菌编码耐热毒素,肠聚集性大肠杆菌,痢疾杆菌spp(非痢疾),Aeromonas spp,Cryptosporidium spp和Entamoeba histolytica增加了死亡风险。在非痢疾性MSD的12-59个月大的儿童中有61例死亡,其中942例志贺氏菌属qPCR阳性儿童中有31例死亡,qPCR阴性的1384例儿童中有30例死亡(HR 2·2,95%CI 1·2– 3·9,p = 0·0090),表明志贺氏菌与死亡风险增加密切相关。
解释
MSD继而降低了LSD的死亡风险。考虑到人口中LSD的病例数是MSD的三倍,因此LSD儿童的死亡人数预计将比MSD的儿童多。由于与LSD相关的主要病原体和与MSD相关的主要病原体是相同的,因此针对这些主要病原体实施疫苗以及快速的诊断和治疗干预措施是合理的投资。
资金
比尔和梅琳达·盖茨基金会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号