Journal of Organometallic Chemistry ( IF 2.3 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.jorganchem.2019.121076 Neslihan Şahin , Nevin Gürbüz , Hande Karabıyık , Hasan Karabıyık , İsmail Özdemir
|
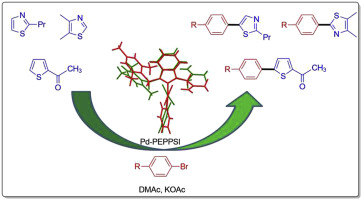
Specific C–H bond can be activated for arylation using aryl halide without the aid of directing the group in the case of electron-rich heteroarenes. The ability to readily generate halo substituted arylated heteroarenes is important in organic chemistry since these species are important building blocks for biochemists. In this manuscript, we report the synthesis of PEPPSI type-novel benzimidazole-based N-heterocyclic carbene-palladium(II) complexes (2a-e). All of the new compounds were fully characterized by 1H, 13C{1H} NMR and FT-IR spectra. The structures of 2c, 2d, and 2e were determined by X-ray crystallography and the prepared complexes (2a-e) were investigated as catalysts for the direct arylation of 2-n-propylthiazole, 4,5-dimethylthiazole and 2-acetylthiophene with various aryl bromides. High catalytic activity for arylation was seen reaction using only 0.5 mol% catalyst for 1 h.
中文翻译:

苯并咪唑基N-杂环卡宾-钯(II)络合物对杂环化合物的丙烯酸化
在富含电子的杂芳烃的情况下,可以使用芳基卤化物活化特定的C H键进行芳基化,而无需指导该基团。容易产生卤素取代的芳基化杂芳烃的能力在有机化学中很重要,因为这些物种是生物化学家的重要组成部分。在此手稿中,我们报告了PEPPSI型新型苯并咪唑基N-杂环卡宾-钯(II)配合物(2a-e)的合成。所有这些新化合物均通过1 H,13 C { 1 H} NMR和FT-IR光谱进行了全面表征。通过X射线晶体学确定2c,2d和2e的结构,并制备配合物(研究了2a-e)作为2-正丙基噻唑,4,5-二甲基噻唑和2-乙酰基噻吩与各种芳基溴直接芳基化的催化剂。仅使用0.5mol%的催化剂反应1小时,观察到高的芳基化催化活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号