当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.actbio.2019.12.020 Wei Liu 1 , Linwei Li 1 , Yuluo Rong 1 , Dingfei Qian 1 , Jian Chen 1 , Zheng Zhou 1 , Yongjun Luo 1 , Dongdong Jiang 1 , Lin Cheng 1 , Shujie Zhao 1 , Fanqi Kong 1 , Jiaxing Wang 1 , Zhimin Zhou 2 , Tao Xu 1 , Fangyi Gong 1 , Yifan Huang 1 , Changjiang Gu 1 , Xuan Zhao 1 , Jianling Bai 3 , Feng Wang 4 , Wene Zhao 4 , Le Zhang 4 , Xiaoyan Li 4 , Guoyong Yin 1 , Jin Fan 1 , Weihua Cai 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.actbio.2019.12.020 Wei Liu 1 , Linwei Li 1 , Yuluo Rong 1 , Dingfei Qian 1 , Jian Chen 1 , Zheng Zhou 1 , Yongjun Luo 1 , Dongdong Jiang 1 , Lin Cheng 1 , Shujie Zhao 1 , Fanqi Kong 1 , Jiaxing Wang 1 , Zhimin Zhou 2 , Tao Xu 1 , Fangyi Gong 1 , Yifan Huang 1 , Changjiang Gu 1 , Xuan Zhao 1 , Jianling Bai 3 , Feng Wang 4 , Wene Zhao 4 , Le Zhang 4 , Xiaoyan Li 4 , Guoyong Yin 1 , Jin Fan 1 , Weihua Cai 1
Affiliation
|
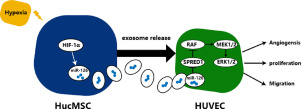
Increasing evidence has suggested that paracrine mechanisms might be involved in the underlying mechanism of mesenchymal stem cells (MSCs) transplantation, and exosomes are an important component of this paracrine role. However, MSCs are usually exposed to normoxia (21% O2) in vitro but experience large differences in oxygen concentration in the body under hypoxia. Indeed, hypoxic precondition of MSCs can enhance their paracrine effects. The main purpose of this study was to determine whether exosomes derived from MSCs under hypoxia (Hypo-Exos) exhibit greater effects on bone fracture healing than those under normoxia (Exos). Using in vivo bone fracture model and in vitro experiments including cell proliferation assay, cell migration assay and so on, we confirmed that Hypo-Exos administration promoted angiogenesis, proliferation and migration to a greater extent when compared to Exos. Furthermore, utilizing a series in vitro and in vivo gain and loss of function experiments, we confirmed a functional role for exosomal miR-126 in the process of bone fracture healing. Meanwhile, we found that knockdown of hypoxia inducible factor 1 (HIF-1α) resulted in a significant decrease of miR-126 in MSCs and exosomes, thereby abolishing the effects of Hypo-Exos. In conclusion, our results demonstrated a mechanism by which Hypo-Exos promote bone fracture healing through exosomal miR-126. Moreover, hypoxia preconditioning mediated enhanced production of exosomal miR-126 through the activation of HIF-1α. Hypoxia preconditioning represents an effective and promising method for the optimization of the therapeutic actions of MSC-derived exosomes for bone fracture healing. STATEMENT OF SIGNIFICANCE: Studies have confirmed that transplantation of exosomes exhibit similar therapeutic effects and functional properties to directly-transplanted stem cells but have less significant adverse effects. However, during in vitro culture conditions, MSCs are usually exposed to normoxia (21% O2) which is very different to the oxygen concentrations found in the body under natural physiological conditions. Our results demonstrated a mechanism by which Hypo-Exos promote bone fracture healing through exosomal miR-126 and the SPRED1/Ras/Erk signaling pathway. Moreover, hypoxia preconditioning mediated enhanced production of exosomal miR-126 through the activation of HIF-1α. Hypoxia preconditioning represents an effective and promising method for the optimization of the therapeutic actions of MSC-derived exosomes for bone fracture healing.
中文翻译:

缺氧的间充质干细胞来源的外泌体通过miR-126的转移促进骨折愈合。
越来越多的证据表明,旁分泌机制可能参与了间充质干细胞(MSCs)移植的潜在机制,并且外泌体是这种旁分泌作用的重要组成部分。但是,MSC通常在体外暴露于常氧(21%O2),但在低氧条件下体内氧浓度存在较大差异。确实,MSC的缺氧前提可以增强其旁分泌作用。这项研究的主要目的是确定在缺氧条件下(Hypo-Exos)衍生自MSC的外泌体是否比在常氧条件下(Exos)表现出对骨折愈合的更大影响。使用体内骨折模型和体外实验(包括细胞增殖测定,细胞迁移测定等),我们证实Hypo-Exos给药可促进血管生成,与Exos相比,扩散和迁移的程度更大。此外,利用一系列体外和体内功能获得和丧失的实验,我们证实了外泌体miR-126在骨折愈合过程中的功能作用。同时,我们发现敲低氧诱导因子1(HIF-1α)导致MSC和外泌体中miR-126的显着降低,从而消除了Hypo-Exos的作用。总之,我们的结果证明了Hypo-Exos通过外泌体miR-126促进骨折愈合的机制。此外,缺氧预处理通过激活HIF-1α介导了外泌体miR-126的产生。缺氧预处理是一种有效且有前途的方法,可用于优化MSC衍生的外泌体在骨折愈合中的治疗作用。重要性声明:研究证实外泌体的移植具有与直接移植的干细胞相似的治疗效果和功能特性,但不良反应较小。但是,在体外培养条件下,MSC通常会暴露于常氧(21%O2),这与自然生理条件下体内的氧气浓度非常不同。我们的结果表明,Hypo-Exos通过外泌体miR-126和SPRED1 / Ras / Erk信号通路促进骨折愈合的机制。此外,缺氧预处理通过激活HIF-1α介导了外泌体miR-126的产生。
更新日期:2019-12-18
中文翻译:

缺氧的间充质干细胞来源的外泌体通过miR-126的转移促进骨折愈合。
越来越多的证据表明,旁分泌机制可能参与了间充质干细胞(MSCs)移植的潜在机制,并且外泌体是这种旁分泌作用的重要组成部分。但是,MSC通常在体外暴露于常氧(21%O2),但在低氧条件下体内氧浓度存在较大差异。确实,MSC的缺氧前提可以增强其旁分泌作用。这项研究的主要目的是确定在缺氧条件下(Hypo-Exos)衍生自MSC的外泌体是否比在常氧条件下(Exos)表现出对骨折愈合的更大影响。使用体内骨折模型和体外实验(包括细胞增殖测定,细胞迁移测定等),我们证实Hypo-Exos给药可促进血管生成,与Exos相比,扩散和迁移的程度更大。此外,利用一系列体外和体内功能获得和丧失的实验,我们证实了外泌体miR-126在骨折愈合过程中的功能作用。同时,我们发现敲低氧诱导因子1(HIF-1α)导致MSC和外泌体中miR-126的显着降低,从而消除了Hypo-Exos的作用。总之,我们的结果证明了Hypo-Exos通过外泌体miR-126促进骨折愈合的机制。此外,缺氧预处理通过激活HIF-1α介导了外泌体miR-126的产生。缺氧预处理是一种有效且有前途的方法,可用于优化MSC衍生的外泌体在骨折愈合中的治疗作用。重要性声明:研究证实外泌体的移植具有与直接移植的干细胞相似的治疗效果和功能特性,但不良反应较小。但是,在体外培养条件下,MSC通常会暴露于常氧(21%O2),这与自然生理条件下体内的氧气浓度非常不同。我们的结果表明,Hypo-Exos通过外泌体miR-126和SPRED1 / Ras / Erk信号通路促进骨折愈合的机制。此外,缺氧预处理通过激活HIF-1α介导了外泌体miR-126的产生。









































 京公网安备 11010802027423号
京公网安备 11010802027423号