当前位置:
X-MOL 学术
›
Adv. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Foamability of aqueous solutions: Role of surfactant type and concentration.
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.cis.2019.102084 B Petkova 1 , S Tcholakova 1 , M Chenkova 1 , K Golemanov 1 , N Denkov 1 , D Thorley 2 , S Stoyanov 3
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.cis.2019.102084 B Petkova 1 , S Tcholakova 1 , M Chenkova 1 , K Golemanov 1 , N Denkov 1 , D Thorley 2 , S Stoyanov 3
Affiliation
|
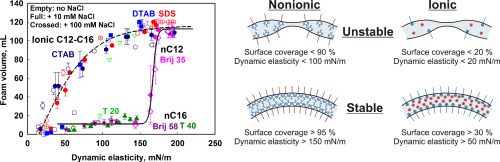
In this paper we study the main surface characteristics which control the foamability of solutions of various surfactants. Systematic series of experiments with anionic, cationic and nonionic surfactants with different head groups and chain lengths are performed in a wide concentration range, from 0.001 mM to 100 mM. The electrolyte (NaCl) concentration is also varied from 0 up to 100 mM. For all surfactants studied, three regions in the dependence of the foamability, VA, on the logarithm of surfactant concentration, lgCS, are observed. In Region 1, VA is very low and depends weakly on CS. In Region 2, VA increases steeply with CS. In Region 3, VA reaches a plateau. To analyse these results, the dynamic and equilibrium surface tensions of the foamed solutions are measured. A key new element in our interpretation of the foaming data is that we use the surface tension measurements to determine the dependence of the main surface properties (surfactant adsorption, surface coverage and surface elasticity) on the surface age of the bubbles. In this way we interpret the results from the foaming tests by considering the properties of the dynamic adsorption layers, formed during foaming. The performed analysis reveals a large qualitative difference between the nonionic and ionic surfactants with respect to their foaming profiles. The data for the nonionic and ionic surfactants merge around two master curves when plotted as a function of the surface coverage, the surface mobility factor, or the Gibbs elasticity of the dynamic adsorption layers. This difference between the ionic and nonionic surfactants is explained with the important contribution of the electrostatic repulsion between the foam film surfaces for the ionic surfactants which stabilizes the dynamic foam films even at moderate surface coverage and at relatively high ionic strength (up to 100 mM). In contrast, the films formed from solutions of nonionic surfactants are stabilized via steric repulsion which becomes sufficiently high to prevent bubble coalescence only at rather high surface coverage (> 90%) which corresponds to related high Gibbs elasticity (> 150 mN/m) and low surface mobility of the dynamic adsorption layers. Mechanistic explanations of all observed trends are provided and some important similarities and differences with the process of emulsification are outlined.
中文翻译:

水溶液的起泡性:表面活性剂类型和浓度的作用。
在本文中,我们研究了控制各种表面活性剂溶液起泡性的主要表面特性。使用具有不同头基和链长的阴离子,阳离子和非离子表面活性剂,在0.001 mM至100 mM的宽浓度范围内进行了系统的一系列实验。电解质(NaCl)的浓度也从0到100 mM不等。对于所有研究的表面活性剂,观察到三个区域,取决于可发泡性VA与表面活性剂浓度lgCS的对数。在区域1中,VA非常低,并且几乎不依赖于CS。在区域2中,VA随CS急剧增加。在区域3中,VA达到平稳状态。为了分析这些结果,测量了泡沫溶液的动态和平衡表面张力。在解释泡沫数据时,一个关键的新要素是我们使用表面张力测量值来确定主要表面特性(表面活性剂吸附,表面覆盖率和表面弹性)与气泡表面寿命的相关性。通过这种方式,我们通过考虑在发泡过程中形成的动态吸附层的特性来解释发泡测试的结果。进行的分析表明,非离子表面活性剂和离子表面活性剂之间在发泡方面存在很大的质量差异。当绘制为动态吸附层的表面覆盖率,表面迁移率因子或吉布斯弹性的函数时,非离子和离子表面活性剂的数据围绕两条主曲线合并。离子型和非离子型表面活性剂之间的这种差异可以解释为泡沫膜表面之间的静电排斥对离子型表面活性剂的重要贡献,即使在中等表面覆盖率和相对较高的离子强度(最高100 mM)下,离子稳定剂也能使动态泡沫膜稳定。相反,由非离子表面活性剂溶液形成的薄膜通过空间排斥作用得以稳定,该空间排斥作用仅在相当高的表面覆盖率(> 90%)时才足以防止气泡聚结,这相当于相关的高吉布斯弹性(> 150 mN / m)和动态吸附层的表面迁移率低。提供了对所有观察到的趋势的机械解释,并概述了与乳化过程的一些重要异同。
更新日期:2019-12-18
中文翻译:

水溶液的起泡性:表面活性剂类型和浓度的作用。
在本文中,我们研究了控制各种表面活性剂溶液起泡性的主要表面特性。使用具有不同头基和链长的阴离子,阳离子和非离子表面活性剂,在0.001 mM至100 mM的宽浓度范围内进行了系统的一系列实验。电解质(NaCl)的浓度也从0到100 mM不等。对于所有研究的表面活性剂,观察到三个区域,取决于可发泡性VA与表面活性剂浓度lgCS的对数。在区域1中,VA非常低,并且几乎不依赖于CS。在区域2中,VA随CS急剧增加。在区域3中,VA达到平稳状态。为了分析这些结果,测量了泡沫溶液的动态和平衡表面张力。在解释泡沫数据时,一个关键的新要素是我们使用表面张力测量值来确定主要表面特性(表面活性剂吸附,表面覆盖率和表面弹性)与气泡表面寿命的相关性。通过这种方式,我们通过考虑在发泡过程中形成的动态吸附层的特性来解释发泡测试的结果。进行的分析表明,非离子表面活性剂和离子表面活性剂之间在发泡方面存在很大的质量差异。当绘制为动态吸附层的表面覆盖率,表面迁移率因子或吉布斯弹性的函数时,非离子和离子表面活性剂的数据围绕两条主曲线合并。离子型和非离子型表面活性剂之间的这种差异可以解释为泡沫膜表面之间的静电排斥对离子型表面活性剂的重要贡献,即使在中等表面覆盖率和相对较高的离子强度(最高100 mM)下,离子稳定剂也能使动态泡沫膜稳定。相反,由非离子表面活性剂溶液形成的薄膜通过空间排斥作用得以稳定,该空间排斥作用仅在相当高的表面覆盖率(> 90%)时才足以防止气泡聚结,这相当于相关的高吉布斯弹性(> 150 mN / m)和动态吸附层的表面迁移率低。提供了对所有观察到的趋势的机械解释,并概述了与乳化过程的一些重要异同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号