当前位置:
X-MOL 学术
›
Mol. Syst. Des. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessing the effect of aromatic residue placement on the α-helical peptide structure and nanofibril formation of 21-mer peptides
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2019/12/17 , DOI: 10.1039/c9me00082h Armin Solemanifar 1, 2, 3, 4 , Tuan A. H. Nguyen 2, 3, 4, 5, 6 , Bronwyn Laycock 1, 2, 3, 4 , Heather M. Shewan 1, 2, 3, 4 , Bogdan C. Donose 1, 2, 3, 4 , Rhiannon C. G. Creasey 1, 2, 3, 4
Molecular Systems Design & Engineering ( IF 3.2 ) Pub Date : 2019/12/17 , DOI: 10.1039/c9me00082h Armin Solemanifar 1, 2, 3, 4 , Tuan A. H. Nguyen 2, 3, 4, 5, 6 , Bronwyn Laycock 1, 2, 3, 4 , Heather M. Shewan 1, 2, 3, 4 , Bogdan C. Donose 1, 2, 3, 4 , Rhiannon C. G. Creasey 1, 2, 3, 4
Affiliation
|
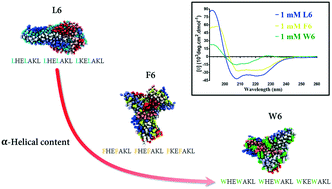
Coiled-coils with defined assembly properties are attractive materials for the manufacture of peptide-based hybrid nanomaterials. In tailoring such peptide assemblies, the incorporation of aromatic residues is increasingly being investigated due to their potential to deliver controllable functionalities, such as interaction with aromatic porphyrins, carbon nanotubes, or graphene. Aromatic residues have the potential to either destabilise or stabilise the α-helical peptide structure, depending on the quantity, type, combination, and position of these residues in the peptide chain. In this work, we used a known synthetic three heptad repeat peptide containing no aromatic residues as an α-helical template. We then substituted the aliphatic residues with two different types of aromatic residues (phenylalanine and tryptophan), varying their number, position, and combination in the peptide chain as a preliminary assessment of the impact on peptide architecture. Circular dichroism (CD) spectroscopy combined with coarse-grained (CG) and all-atom (AA) molecular dynamics (MD) simulations was used to analyse the peptide structure and assembly. Aromatic residues that were designed to be within the hydrophobic core had more impact on self-assembly than those placed on the outer face of the coil. Tryptophan was seen to destabilise the α-helical structure more than phenylalanine, potentially due to steric hindrance and hydrogen-bonding interactions. Using atomic force microscopy (AFM) and supported by CG-MD simulation, substituting all phenylalanine residues with tryptophan appeared to completely destabilise fibril-formation propensity. Substituting tryptophan into the first heptad repeat was seen to have a greater impact on fibril formation compared to substitution into the third heptad repeat, suggesting the importance of sequence design. These results add to the body of knowledge used to inform the design of α-helical peptides when incorporating aromatic residues.
中文翻译:

评估芳族残基位置对21-mer肽的α-螺旋肽结构和纳米原纤维形成的影响
具有确定的组装特性的卷形线圈是用于制造基于肽的杂化纳米材料的有吸引力的材料。在定制这样的肽装配体中,由于芳香族残基具有传递可控功能的潜力,例如与芳香族卟啉,碳纳米管或石墨烯的相互作用,因此越来越多地对其进行研究。芳族残基具有使α-螺旋肽结构不稳定或稳定的潜力,具体取决于这些残基在肽链中的数量,类型,组合和位置。在这项工作中,我们使用不含芳族残基的已知合成三七重复序列肽作为α-螺旋模板。然后,我们用两种不同类型的芳香族残基(苯丙氨酸和色氨酸)取代脂肪族残基,改变它们的数量,位置以及在肽链中的组合,作为对肽结构影响的初步评估。圆二色谱(CD)光谱结合粗粒度(CG)和全原子(AA)分子动力学(MD)模拟用于分析肽的结构和组装。被设计为位于疏水核内的芳族残基对自组装的影响要大于位于线圈外表面的那些。人们发现色氨酸比苯丙氨酸更容易破坏α螺旋结构的稳定性,这可能是由于位阻和氢键相互作用所致。使用原子力显微镜(AFM)并得到CG-MD模拟的支持,用色氨酸取代所有苯丙氨酸残基似乎完全破坏了原纤维形成的倾向。与将色氨酸替换为第三庚肽重复序列相比,将色氨酸替换为第一庚肽重复序列对原纤维形成的影响更大。这些结果增加了在掺入芳族残基时用于告知α-螺旋肽设计的知识体系。
更新日期:2020-02-24
中文翻译:

评估芳族残基位置对21-mer肽的α-螺旋肽结构和纳米原纤维形成的影响
具有确定的组装特性的卷形线圈是用于制造基于肽的杂化纳米材料的有吸引力的材料。在定制这样的肽装配体中,由于芳香族残基具有传递可控功能的潜力,例如与芳香族卟啉,碳纳米管或石墨烯的相互作用,因此越来越多地对其进行研究。芳族残基具有使α-螺旋肽结构不稳定或稳定的潜力,具体取决于这些残基在肽链中的数量,类型,组合和位置。在这项工作中,我们使用不含芳族残基的已知合成三七重复序列肽作为α-螺旋模板。然后,我们用两种不同类型的芳香族残基(苯丙氨酸和色氨酸)取代脂肪族残基,改变它们的数量,位置以及在肽链中的组合,作为对肽结构影响的初步评估。圆二色谱(CD)光谱结合粗粒度(CG)和全原子(AA)分子动力学(MD)模拟用于分析肽的结构和组装。被设计为位于疏水核内的芳族残基对自组装的影响要大于位于线圈外表面的那些。人们发现色氨酸比苯丙氨酸更容易破坏α螺旋结构的稳定性,这可能是由于位阻和氢键相互作用所致。使用原子力显微镜(AFM)并得到CG-MD模拟的支持,用色氨酸取代所有苯丙氨酸残基似乎完全破坏了原纤维形成的倾向。与将色氨酸替换为第三庚肽重复序列相比,将色氨酸替换为第一庚肽重复序列对原纤维形成的影响更大。这些结果增加了在掺入芳族残基时用于告知α-螺旋肽设计的知识体系。











































 京公网安备 11010802027423号
京公网安备 11010802027423号