Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N-Glycosylation profiling of intact target proteins by high-resolution mass spectrometry (MS) and glycan analysis using ion mobility-MS/MS.
Analyst ( IF 3.6 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9an02081k Alessandro Quaranta 1 , Maya Spasova 1 , Elena Passarini 1 , Isabella Karlsson 1 , Lorena Ndreu 1 , Gunnar Thorsén 2 , Leopold L Ilag 1
Analyst ( IF 3.6 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9an02081k Alessandro Quaranta 1 , Maya Spasova 1 , Elena Passarini 1 , Isabella Karlsson 1 , Lorena Ndreu 1 , Gunnar Thorsén 2 , Leopold L Ilag 1
Affiliation
|
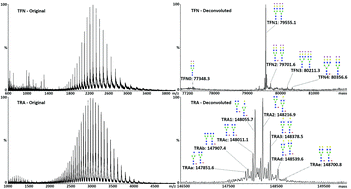
Glycosylation influences the structure and functionality of glycoproteins, and is regulated by genetic and environmental factors. The types and abundance of glycans on glycoproteins can vary due to diseases such as cancer, inflammation, autoimmune and neurodegenerative disorders. Due to the crucial role glycans play in modulating protein function, glycosylation analysis could lead to the discovery of novel biomarkers and is of prime importance in controlling the quality of glycoprotein biopharmaceuticals. Here, we present a method for the identification and quantification of glycoforms directly on intact proteins, after immunoaffinity purification from biological fluids. The method was validated and applied to serum transferrin and the biopharmaceutical trastuzumab. The accuracy of the method, expressed as the relative error (RE), ranged from 2.1 (at high concentrations) to 7.9% (at low concentrations), and intra- and inter-day precision, expressed as relative standard deviation (RSD), was 3.2 and 8.2%, respectively. The sensitivity and linearity of the method were suitable for serum analysis and the LOQ was calculated to be 3.1 and 4.4 μg mL-1 for transferrin (TFN) and trastuzumab (TRA), respectively. Its application to transferrin from five healthy human serum samples yielded concentrations between 1.61 and 3.17 mg mL-1, which are in agreement with blood reference levels. In parallel, the structure of the identified glycans was determined by ion mobility spectrometry coupled with tandem mass spectrometry. No chromatographic separation was required and sample preparation was performed in a semi-automatic manner, facilitating the handling of up to 12 samples at a time. This method should be useful for clinical laboratories and for the quality control of large batches of biopharmaceuticals.
中文翻译:

通过高分辨率质谱(MS)和使用离子迁移率MS / MS进行的聚糖分析,对完整的目标蛋白进行N-糖基化分析。
糖基化影响糖蛋白的结构和功能,并受遗传和环境因素的调节。糖蛋白上聚糖的类型和丰度可能会因疾病(例如癌症,炎症,自身免疫性疾病和神经退行性疾病)而有所不同。由于聚糖在调节蛋白质功能中起关键作用,糖基化分析可能会导致发现新的生物标记物,并且在控制糖蛋白生物药物的质量方面至关重要。在这里,我们提出了一种从生物体液中进行免疫亲和纯化后,直接在完整蛋白上鉴定和定量糖型的方法。该方法经过验证,可用于血清转铁蛋白和生物制药曲妥珠单抗。该方法的精度表示为相对误差(RE),范围为2。1(在高浓度下)至7.9%(在低浓度下),并且日内和日间精度(以相对标准偏差(RSD)表示)分别为3.2%和8.2%。该方法的灵敏度和线性适用于血清分析,计算得出转铁蛋白(TFN)和曲妥珠单抗(TRA)的LOQ分别为3.1和4.4μgmL-1。应用于五种健康人血清样本中的转铁蛋白,其浓度在1.61至3.17 mg mL-1之间,与血液参考水平相符。平行地,通过离子迁移谱结合串联质谱法确定所鉴定的聚糖的结构。无需色谱分离,并且以半自动方式进行样品制备,从而一次最多可处理12个样品。
更新日期:2020-03-03
中文翻译:

通过高分辨率质谱(MS)和使用离子迁移率MS / MS进行的聚糖分析,对完整的目标蛋白进行N-糖基化分析。
糖基化影响糖蛋白的结构和功能,并受遗传和环境因素的调节。糖蛋白上聚糖的类型和丰度可能会因疾病(例如癌症,炎症,自身免疫性疾病和神经退行性疾病)而有所不同。由于聚糖在调节蛋白质功能中起关键作用,糖基化分析可能会导致发现新的生物标记物,并且在控制糖蛋白生物药物的质量方面至关重要。在这里,我们提出了一种从生物体液中进行免疫亲和纯化后,直接在完整蛋白上鉴定和定量糖型的方法。该方法经过验证,可用于血清转铁蛋白和生物制药曲妥珠单抗。该方法的精度表示为相对误差(RE),范围为2。1(在高浓度下)至7.9%(在低浓度下),并且日内和日间精度(以相对标准偏差(RSD)表示)分别为3.2%和8.2%。该方法的灵敏度和线性适用于血清分析,计算得出转铁蛋白(TFN)和曲妥珠单抗(TRA)的LOQ分别为3.1和4.4μgmL-1。应用于五种健康人血清样本中的转铁蛋白,其浓度在1.61至3.17 mg mL-1之间,与血液参考水平相符。平行地,通过离子迁移谱结合串联质谱法确定所鉴定的聚糖的结构。无需色谱分离,并且以半自动方式进行样品制备,从而一次最多可处理12个样品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号