当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the Electron-Transfer Regime of Peroxydisulfate Activation on Carbon Nanotubes: The Role of Oxygen Functional Groups.
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.est.9b06208 Wei Ren 1, 2 , Liangliang Xiong 1 , Gang Nie 1, 2 , Hui Zhang 1 , Xiaoguang Duan 2 , Shaobin Wang 2
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.est.9b06208 Wei Ren 1, 2 , Liangliang Xiong 1 , Gang Nie 1, 2 , Hui Zhang 1 , Xiaoguang Duan 2 , Shaobin Wang 2
Affiliation
|
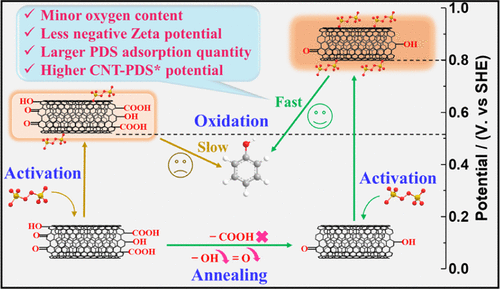
Carbon-driven advanced oxidation processes are appealing in wastewater purification because of the metal-free feature of the carbocatalysts. However, the regime of the emerging nonradical pathway is ambiguous because of the intricate carbon structure. To this end, this study was dedicated to unveil the intrinsic structure-performance relationship of peroxydisulfate (PDS) activation by carbon nanotubes (CNTs) toward nonradical oxidation of organics such as phenol (PE) via electron transfer. Eighteen analogical CNTs were synthesized and functionalized with different categories and contents of oxygen species. The quenching tests and chronopotentiometry suggest that an improved reactivity of surface-regulated CNTs was attributed to the reinforced electron-transfer regime without generation of free radicals and singlet oxygen. The quantitative structure-activity relationships were established and correlated to the Tafel equation, which unveils the nature of the nonradical oxidation by CNT-activated PDS complexes (CNT-PDS*). First, a decline in the concentration of oxygen groups in CNTs will make the zeta potential of the CNT become less negative in neutral solutions, which facilitated the adsorption of PDS because of weaker electrostatic repulsion. Then, the metastable CNT-PDS* was formed, which elevated the oxidation capacity of the CNT. Finally, PE would be oxidized over CNT-PDS* via electron transfer to fulfill the redox cycle. Moreover, the nonradical oxidation rate was uncovered to be exponentially related with the potential of the complexes, suggesting that the nonradical oxidation by the CNT-PDS* undergoes a mechanism analogous to anodic oxidation.
中文翻译:

碳纳米管上过二硫酸盐活化的电子转移机制的见解:氧官能团的作用。
由于碳催化剂的无金属特性,碳驱动的高级氧化工艺在废水净化中很有吸引力。但是,由于复杂的碳结构,新兴的非自由基途径的机制尚不明确。为此,本研究致力于揭示碳纳米管(CNT)活化过氧二硫酸盐(PDS)通过电子转移对有机物(例如苯酚(PE))进行非自由基氧化的内在结构与性能之间的关系。合成了十八种类似的碳纳米管,并用不同种类和含量的氧进行了功能化。猝灭测试和计时电位法表明,表面调节的CNTs的反应性提高归因于增强的电子转移机制,而没有自由基和单线态氧的产生。建立了定量的构效关系,并将其与Tafel方程关联,该方程揭示了CNT活化的PDS络合物(CNT-PDS *)的非自由基氧化性质。首先,碳纳米管中氧原子浓度的下降将使碳纳米管的ζ电势在中性溶液中的负电性降低,这由于静电斥力较弱而促进了PDS的吸附。然后,形成了亚稳态的CNT-PDS *,这提高了CNT的氧化能力。最后,PE将通过电子转移在CNT-PDS *上氧化,以完成氧化还原循环。此外,未发现非自由基氧化速率与配合物的电势呈指数关系,表明CNT-PDS *的非自由基氧化经历了类似于阳极氧化的机理。
更新日期:2019-12-30
中文翻译:

碳纳米管上过二硫酸盐活化的电子转移机制的见解:氧官能团的作用。
由于碳催化剂的无金属特性,碳驱动的高级氧化工艺在废水净化中很有吸引力。但是,由于复杂的碳结构,新兴的非自由基途径的机制尚不明确。为此,本研究致力于揭示碳纳米管(CNT)活化过氧二硫酸盐(PDS)通过电子转移对有机物(例如苯酚(PE))进行非自由基氧化的内在结构与性能之间的关系。合成了十八种类似的碳纳米管,并用不同种类和含量的氧进行了功能化。猝灭测试和计时电位法表明,表面调节的CNTs的反应性提高归因于增强的电子转移机制,而没有自由基和单线态氧的产生。建立了定量的构效关系,并将其与Tafel方程关联,该方程揭示了CNT活化的PDS络合物(CNT-PDS *)的非自由基氧化性质。首先,碳纳米管中氧原子浓度的下降将使碳纳米管的ζ电势在中性溶液中的负电性降低,这由于静电斥力较弱而促进了PDS的吸附。然后,形成了亚稳态的CNT-PDS *,这提高了CNT的氧化能力。最后,PE将通过电子转移在CNT-PDS *上氧化,以完成氧化还原循环。此外,未发现非自由基氧化速率与配合物的电势呈指数关系,表明CNT-PDS *的非自由基氧化经历了类似于阳极氧化的机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号