当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revealing Conformational Transitions in G-Protein-Coupled Receptor Rhodopsin upon Phosphorylation.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.biochem.9b00884 Nidhi Jatana 1 , S Keerthic Aswin 1 , Surabhi Rathore 1, 2 , Lipi Thukral 1, 2, 3
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-17 , DOI: 10.1021/acs.biochem.9b00884 Nidhi Jatana 1 , S Keerthic Aswin 1 , Surabhi Rathore 1, 2 , Lipi Thukral 1, 2, 3
Affiliation
|
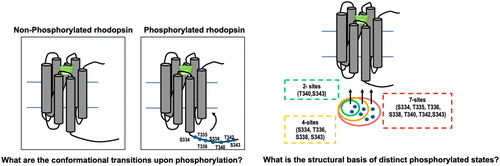
G-protein-coupled receptors (GPCRs) have evolved as highly specialized cellular machinery that can dictate biological outcomes in response to diverse stimuli. Specifically, they induce multiple pathway responses upon structural perturbations induced at local protein sites. GPCRs utilize a concurrent strategy involving a central transmembrane topology and biochemical modifications for precise functional implementation. However, the specific role of the latter is not known due to the lack of precise probing techniques that can characterize receptor dynamics upon biochemical modifications. Phosphorylation is known to be one of the critical biochemical modifications in GPCRs that aids in receptor desensitization via arrestin binding. Here, we carry out all-atom molecular dynamics simulations of rhodopsin in a membrane environment to study its conformational dynamics induced upon phosphorylation. Interestingly, our comparative analysis of non-phosphorylated and phosphorylated rhodopsin structure demonstrated enhanced receptor stability upon phosphorylation at the C-terminal region that leads to the opening of the extracellular part of the transmembrane helices. In addition, monitoring the distinct number of phosphorylation states showed that having fewer phosphorylated residues does not bring about appropriate conformational changes in the extracellular region. Since phosphorylation results in receptor desensitization and recycling of the ligand, our findings provide significant insights into the conformational dynamics of the mechanism of ligand exit from the receptor.
中文翻译:

揭示磷酸化后G蛋白偶联受体视紫红质的构象转变。
G蛋白偶联受体(GPCR)已发展成为高度专业化的细胞机制,可以决定对各种刺激作出反应的生物学结果。具体而言,它们在局部蛋白质位点诱导的结构扰动下诱导多途径反应。GPCR利用并发策略,涉及中央跨膜拓扑结构和生化修饰,以实现精确的功能实现。然而,由于缺乏可表征生化修饰后受体动态的精确探测技术,后者的具体作用尚不清楚。已知磷酸化是GPCR中的关键生物化学修饰之一,可通过抑制素结合来帮助受体脱敏。这里,我们在膜环境中进行了视紫红质的全原子分子动力学模拟,以研究其在磷酸化作用下诱导的构象动力学。有趣的是,我们对非磷酸化和磷酸化视紫红质结构的比较分析表明,在C端区域发生磷酸化后,受体稳定性增强,从而导致跨膜螺旋的胞外部分打开。另外,监测不同数量的磷酸化状态表明,具有较少的磷酸化残基不会在细胞外区域引起适当的构象变化。由于磷酸化导致受体脱敏和配体的再循环,我们的发现为配体从受体中退出的构象动力学提供了重要的见识。
更新日期:2019-12-27
中文翻译:

揭示磷酸化后G蛋白偶联受体视紫红质的构象转变。
G蛋白偶联受体(GPCR)已发展成为高度专业化的细胞机制,可以决定对各种刺激作出反应的生物学结果。具体而言,它们在局部蛋白质位点诱导的结构扰动下诱导多途径反应。GPCR利用并发策略,涉及中央跨膜拓扑结构和生化修饰,以实现精确的功能实现。然而,由于缺乏可表征生化修饰后受体动态的精确探测技术,后者的具体作用尚不清楚。已知磷酸化是GPCR中的关键生物化学修饰之一,可通过抑制素结合来帮助受体脱敏。这里,我们在膜环境中进行了视紫红质的全原子分子动力学模拟,以研究其在磷酸化作用下诱导的构象动力学。有趣的是,我们对非磷酸化和磷酸化视紫红质结构的比较分析表明,在C端区域发生磷酸化后,受体稳定性增强,从而导致跨膜螺旋的胞外部分打开。另外,监测不同数量的磷酸化状态表明,具有较少的磷酸化残基不会在细胞外区域引起适当的构象变化。由于磷酸化导致受体脱敏和配体的再循环,我们的发现为配体从受体中退出的构象动力学提供了重要的见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号