当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulating ALS-Related Amyloidogenic TDP-43307-319 Oligomeric Aggregates with Computationally Derived Therapeutic Molecules.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.biochem.9b00905 Veronica Laos 1 , Dezmond Bishop 1 , Christian A Lang 2 , Nicole M Marsh 3 , Kristi Lazar Cantrell 3 , Steven K Buratto 1 , Ambuj K Singh 4 , Michael T Bowers 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-26 , DOI: 10.1021/acs.biochem.9b00905 Veronica Laos 1 , Dezmond Bishop 1 , Christian A Lang 2 , Nicole M Marsh 3 , Kristi Lazar Cantrell 3 , Steven K Buratto 1 , Ambuj K Singh 4 , Michael T Bowers 1
Affiliation
|
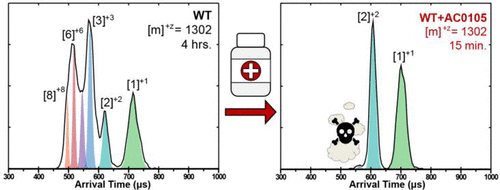
TDP-43 aggregates are a salient feature of amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), and a variety of other neurodegenerative diseases, including Alzheimer's disease (AD). With an anticipated growth in the most susceptible demographic, projections predict neurodegenerative diseases will potentially affect 15 million people in the United States by 2050. Currently, there are no cures for ALS, FTD, or AD. Previous studies of the amyloidogenic core of TDP-43 have demonstrated that oligomers greater than a trimer are associated with toxicity. Utilizing a joint pharmacophore space (JPS) method, potential drugs have been designed specifically for amyloid-related diseases. These molecules were generated on the basis of key chemical features necessary for blood-brain barrier permeability, low adverse side effects, and target selectivity. Combining ion-mobility mass spectrometry and atomic force microscopy with the JPS computational method allows us to more efficiently evaluate a potential drug's efficacy in disrupting the development of putative toxic species. Our results demonstrate the dissociation of higher-order oligomers in the presence of these novel JPS-generated inhibitors into smaller oligomer species. Additionally, drugs approved by the Food and Drug Administration for the treatment of ALS were also evaluated and demonstrated to maintain higher-order oligomeric assemblies. Possible mechanisms for the observed action of the JPS molecules are discussed.
中文翻译:

用计算派生的治疗分子调节ALS相关的淀粉样蛋白生成的TDP-43307-319寡聚聚集体。
TDP-43聚集体是肌萎缩性侧索硬化症(ALS),额颞叶痴呆(FTD)以及多种其他神经退行性疾病(包括阿尔茨海默氏病(AD))的显着特征。随着最易感人群的预期增长,预测预测到2050年,神经退行性疾病将在美国影响1500万人。目前,尚无治愈ALS,FTD或AD的方法。TDP-43的淀粉样蛋白生成核心的先前研究表明,大于三聚体的寡聚体与毒性有关。利用联合药效团空间(JPS)方法,已专门设计了针对淀粉样蛋白相关疾病的潜在药物。这些分子是根据血脑屏障通透性,低不良副作用,和目标选择性。将离子淌度质谱和原子力显微镜与JPS计算方法结合使用,使我们能够更有效地评估潜在药物在破坏假定毒性物种发展方面的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。在破坏假定的有毒物种发展中的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。在破坏假定的有毒物种发展中的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。
更新日期:2019-12-27
中文翻译:

用计算派生的治疗分子调节ALS相关的淀粉样蛋白生成的TDP-43307-319寡聚聚集体。
TDP-43聚集体是肌萎缩性侧索硬化症(ALS),额颞叶痴呆(FTD)以及多种其他神经退行性疾病(包括阿尔茨海默氏病(AD))的显着特征。随着最易感人群的预期增长,预测预测到2050年,神经退行性疾病将在美国影响1500万人。目前,尚无治愈ALS,FTD或AD的方法。TDP-43的淀粉样蛋白生成核心的先前研究表明,大于三聚体的寡聚体与毒性有关。利用联合药效团空间(JPS)方法,已专门设计了针对淀粉样蛋白相关疾病的潜在药物。这些分子是根据血脑屏障通透性,低不良副作用,和目标选择性。将离子淌度质谱和原子力显微镜与JPS计算方法结合使用,使我们能够更有效地评估潜在药物在破坏假定毒性物种发展方面的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。在破坏假定的有毒物种发展中的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。在破坏假定的有毒物种发展中的功效。我们的结果证明,在这些新型JPS生成的抑制剂存在下,高阶低聚物会解离为较小的低聚物。此外,还对食品和药物管理局批准用于治疗ALS的药物进行了评估,并证明其可维持更高阶的寡聚体组装体。讨论了JPS分子观察到的作用的可能机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号