当前位置:
X-MOL 学术
›
Eur. J. Pharm. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetics, pharmacodynamics and safety of a novel extrafine BDP/FF/GB combination delivered via metered-dose inhaler in healthy Chinese subjects.
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.ejps.2019.105198 Chao Hu 1 , Jia Miao 1 , Shiqing Shu 1 , Ying Wang 1 , Xiaohong Zhu 1 , Zhu Luo 1
European Journal of Pharmaceutical Sciences ( IF 4.3 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.ejps.2019.105198 Chao Hu 1 , Jia Miao 1 , Shiqing Shu 1 , Ying Wang 1 , Xiaohong Zhu 1 , Zhu Luo 1
Affiliation
|
BACKGROUND
BDP/FF/GB pMDI is a novel triple fixed-dose combination of extra-fine inhalation aerosol beclomethasone dipropionate (BDP)/formoterol fumarate (FF)/glycopyrronium bromide (GB). Limited data on the pharmacokinetic (PK) and pharmacodynamic (PD) properties of BDP/FF/GB fixed-dose combination in healthy subjects was available.
PURPOSES
This study aimed to evaluate the pharmacokinetics, pharmacodynamics and safety of BDP/FF/GB pMDI in healthy Chinese subjects.
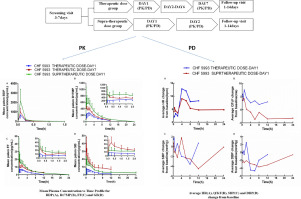
METHODS
This is an open-label, parallel-group, randomized, single and multiple dose study. In the single dose group, subjects received single supra-therapeutic inhaled dose of BDP/FF/GB pMDI (BDP/FF/GB 400/24/50 µg). In the multiple dose group, subjects received therapeutic inhaled dose of BDP/FF/GB pMDI (BDP/FF/GB 200/12/25 µg), twice daily, for 7 consecutive days. Plasma BDP, B17MP, formoterol and GB were determined by a validated ultra performance liquid chromatography method with tandem mass spectrometric detection (UPLC/MS-MS). Heart rate (HR), QTcF, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were evaluated as the surrogate indicators of pharmacodynamic effects.
RESULTS
A total of 24 subjects were randomized and 22 (11 in each group) completed the study. The dose adjusted pharmacokinetic profiles of BDP, beclomethasone-17-monopropionate (B17MP, the most active metabolite of BDP), formoterol and GB were overall similar in therapeutic and supra- therapeutic dose group, showing dose proportional increase of the systemic exposure to BDP, B17MP, formoterol and GB. The pharmacodynamic variables were within the normal range and showed no significant difference between the two groups. All the treatment-emergent adverse events (TEAEs) were mild and no severe TEAE was reported.
CONCLUSIONS
Dose adjusted PK profiles were similar between therapeutic and supra-therapeutic dose for all compounds, nearly dose proportional systemic exposure to B17MP, formoterol and GB after BDP/FF/GB pMDI administration in healthy Chinese subjects. BDP/FF/GB pMDI was safe and well tolerated in healthy Chinese subjects. The PK profiles were comparable to previously published data from Western European healthy Caucasian subjects.
更新日期:2019-12-18











































 京公网安备 11010802027423号
京公网安备 11010802027423号