Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Structural Basis for Low Conductance in the Membrane Protein VDAC upon β-NADH Binding and Voltage Gating.
Structure ( IF 4.4 ) Pub Date : 2019-12-10 , DOI: 10.1016/j.str.2019.11.015 Raphael Böhm 1 , Giuseppe Federico Amodeo 2 , Sruthi Murlidaran 3 , Shashank Chavali 4 , Gerhard Wagner 5 , Mathias Winterhalter 2 , Grace Brannigan 6 , Sebastian Hiller 1
Structure ( IF 4.4 ) Pub Date : 2019-12-10 , DOI: 10.1016/j.str.2019.11.015 Raphael Böhm 1 , Giuseppe Federico Amodeo 2 , Sruthi Murlidaran 3 , Shashank Chavali 4 , Gerhard Wagner 5 , Mathias Winterhalter 2 , Grace Brannigan 6 , Sebastian Hiller 1
Affiliation
|
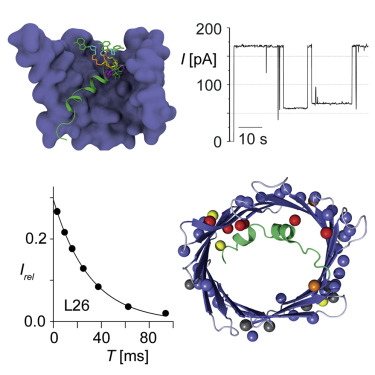
The voltage-dependent anion channel (VDAC) forms the primary diffusion pore of the outer mitochondrial membrane. In its apo form, VDAC adopts an open conformation with high conductance. States of lower conductance can be induced by ligand binding or the application of voltage. Here, we clarify at the atomic level how β-NADH binding leads to a low-conductance state and characterize the role of the VDAC N-terminal helix in voltage gating. A high-resolution NMR structure of human VDAC-1 with bound NADH, combined with molecular dynamics simulation show that β-NADH binding reduces the pore conductance sterically without triggering a structural change. Electrophysiology recordings of crosslinked protein variants and NMR relaxation experiments probing different time scales show that increased helix dynamics is present in the open state and that motions of the N-terminal helices are involved in the VDAC voltage gating mechanism.
中文翻译:

β-NADH 结合和电压门控时膜蛋白 VDAC 低电导的结构基础。
电压依赖性阴离子通道(VDAC)形成线粒体外膜的初级扩散孔。在其apo形式中,VDAC采用具有高电导的开放构象。较低电导的状态可以通过配体结合或施加电压来诱导。在这里,我们在原子水平上阐明了 β-NADH 结合如何导致低电导状态,并表征了 VDAC N 端螺旋在电压门控中的作用。结合了 NADH 的人 VDAC-1 的高分辨率 NMR 结构,结合分子动力学模拟表明,β-NADH 结合在空间上降低了孔导率,而不会引发结构变化。交联蛋白变体的电生理学记录和探测不同时间尺度的 NMR 弛豫实验表明,开放状态下螺旋动力学增加,并且 N 端螺旋的运动参与 VDAC 电压门控机制。
更新日期:2019-12-18
中文翻译:

β-NADH 结合和电压门控时膜蛋白 VDAC 低电导的结构基础。
电压依赖性阴离子通道(VDAC)形成线粒体外膜的初级扩散孔。在其apo形式中,VDAC采用具有高电导的开放构象。较低电导的状态可以通过配体结合或施加电压来诱导。在这里,我们在原子水平上阐明了 β-NADH 结合如何导致低电导状态,并表征了 VDAC N 端螺旋在电压门控中的作用。结合了 NADH 的人 VDAC-1 的高分辨率 NMR 结构,结合分子动力学模拟表明,β-NADH 结合在空间上降低了孔导率,而不会引发结构变化。交联蛋白变体的电生理学记录和探测不同时间尺度的 NMR 弛豫实验表明,开放状态下螺旋动力学增加,并且 N 端螺旋的运动参与 VDAC 电压门控机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号