Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Tuning of the Axonal Mitochondrial Ca2+ Uniporter Ensures Metabolic Flexibility of Neurotransmission.
Neuron ( IF 14.7 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.neuron.2019.11.020 Ghazaleh Ashrafi 1 , Jaime de Juan-Sanz 1 , Ryan J Farrell 2 , Timothy A Ryan 1
Neuron ( IF 14.7 ) Pub Date : 2019-12-17 , DOI: 10.1016/j.neuron.2019.11.020 Ghazaleh Ashrafi 1 , Jaime de Juan-Sanz 1 , Ryan J Farrell 2 , Timothy A Ryan 1
Affiliation
|
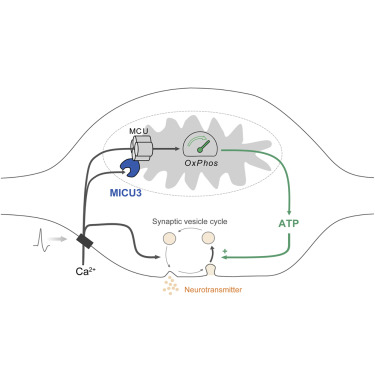
The brain is a vulnerable metabolic organ and must adapt to different fuel conditions to sustain function. Nerve terminals are a locus of this vulnerability, but how they regulate ATP synthesis as fuel conditions vary is unknown. We show that synapses can switch from glycolytic to oxidative metabolism, but to do so, they rely on activity-driven presynaptic mitochondrial Ca2+ uptake to accelerate ATP production. We demonstrate that, whereas mitochondrial Ca2+ uptake requires elevated extramitochondrial Ca2+ in non-neuronal cells, axonal mitochondria readily take up Ca2+ in response to small changes in external Ca2+. We identified the brain-specific protein MICU3 as a critical driver of this tuning of Ca2+ sensitivity. Ablation of MICU3 renders axonal mitochondria similar to non-neuronal mitochondria, prevents acceleration of local ATP synthesis, and impairs presynaptic function under oxidative conditions. Thus, presynaptic mitochondria rely on MICU3 to facilitate mitochondrial Ca2+ uptake during activity and achieve metabolic flexibility.
中文翻译:

轴突线粒体Ca2 + Uniporter的分子调节可确保神经传递的代谢灵活性。
大脑是脆弱的新陈代谢器官,必须适应不同的燃料条件以维持功能。神经终端是该漏洞的一个源头,但随着燃料条件的变化,它们如何调节ATP的合成尚不清楚。我们显示突触可以从糖酵解转换为氧化代谢,但是要这样做,它们依赖于活性驱动的突触前线粒体Ca2 +摄取来加速ATP的产生。我们证明,虽然线粒体Ca2 +吸收需要在非神经元细胞中升高线粒体Ca2 +,但轴突线粒体很容易吸收Ca2 +,以响应外部Ca2 +的微小变化。我们确定了大脑特异性蛋白MICU3是这种调节Ca2 +敏感性的关键驱动因素。MICU3的消融使轴突线粒体类似于非神经元的线粒体,阻止了局部ATP合成的加速,并在氧化条件下削弱突触前功能。因此,突触前线粒体在活动期间依靠MICU3促进线粒体Ca2 +的摄取并实现代谢的灵活性。
更新日期:2019-12-18
中文翻译:

轴突线粒体Ca2 + Uniporter的分子调节可确保神经传递的代谢灵活性。
大脑是脆弱的新陈代谢器官,必须适应不同的燃料条件以维持功能。神经终端是该漏洞的一个源头,但随着燃料条件的变化,它们如何调节ATP的合成尚不清楚。我们显示突触可以从糖酵解转换为氧化代谢,但是要这样做,它们依赖于活性驱动的突触前线粒体Ca2 +摄取来加速ATP的产生。我们证明,虽然线粒体Ca2 +吸收需要在非神经元细胞中升高线粒体Ca2 +,但轴突线粒体很容易吸收Ca2 +,以响应外部Ca2 +的微小变化。我们确定了大脑特异性蛋白MICU3是这种调节Ca2 +敏感性的关键驱动因素。MICU3的消融使轴突线粒体类似于非神经元的线粒体,阻止了局部ATP合成的加速,并在氧化条件下削弱突触前功能。因此,突触前线粒体在活动期间依靠MICU3促进线粒体Ca2 +的摄取并实现代谢的灵活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号