当前位置:
X-MOL 学术
›
Lancet Diabetes Endocrinol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of professional-mode flash glucose monitoring, at 3-month intervals, in adults with type 2 diabetes in general practice (GP-OSMOTIC): a pragmatic, open-label, 12-month, randomised controlled trial.
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2020-01-01 , DOI: 10.1016/s2213-8587(19)30385-7 John Furler 1 , David O'Neal 2 , Jane Speight 3 , Irene Blackberry 4 , Jo-Anne Manski-Nankervis 1 , Sharmala Thuraisingam 1 , Katie de La Rue 1 , Louise Ginnivan 1 , Rebecca Doyle 1 , Elizabeth Holmes-Truscott 3 , Kamlesh Khunti 5 , Kim Dalziel 6 , Jason Chiang 1 , Ralph Audehm 1 , Mark Kennedy 1 , Malcolm Clark 1 , Alicia Jenkins 7 , Amelia J Lake 3 , Andrzej S Januszewski 7 , Max Catchpool 6 , Danny Liew 8 , Philip Clarke 6 , James Best 9
The Lancet Diabetes & Endocrinology ( IF 44.0 ) Pub Date : 2020-01-01 , DOI: 10.1016/s2213-8587(19)30385-7 John Furler 1 , David O'Neal 2 , Jane Speight 3 , Irene Blackberry 4 , Jo-Anne Manski-Nankervis 1 , Sharmala Thuraisingam 1 , Katie de La Rue 1 , Louise Ginnivan 1 , Rebecca Doyle 1 , Elizabeth Holmes-Truscott 3 , Kamlesh Khunti 5 , Kim Dalziel 6 , Jason Chiang 1 , Ralph Audehm 1 , Mark Kennedy 1 , Malcolm Clark 1 , Alicia Jenkins 7 , Amelia J Lake 3 , Andrzej S Januszewski 7 , Max Catchpool 6 , Danny Liew 8 , Philip Clarke 6 , James Best 9
Affiliation
|
BACKGROUND
Continuous glucose monitoring, either real-time (personal) or retrospective (professional mode), can identify day-to-day glucose profiles to guide management decisions for people with type 2 diabetes. We aimed to examine the effects of professional-mode flash glucose monitoring, done at 3-month intervals, in adults with type 2 diabetes in general practice.
METHODS
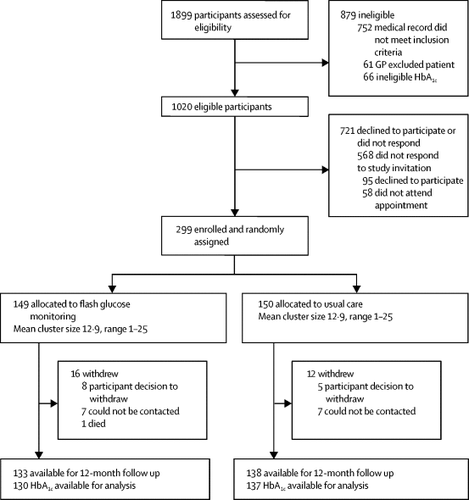
We did a pragmatic, two-arm, open label, 12-month, individually randomised controlled trial (GP-OSMOTIC) in 25 general practices in Victoria, Australia. Eligible participants were adults aged 18-80 years, with type 2 diabetes diagnosed for at least 1 year and HbA1c at least 5·5 mmol/mol (0·5%) above their target in the past month despite being prescribed at least two non-insulin glucose-lowering drugs, insulin, or both (with therapy stable for at least 4 months). We randomly assigned participants (1:1) to either use of a professional-mode flash glucose monitoring system or usual clinical care (control). All participants wore the flash glucose monitoring sensor at baseline, and electronic randomisation (using permuted block sizes of four and six, and stratified by clinic) was done after the sensor was attached. Masking of participants and treating clinicians to group allocation was not possible, but the study statistician was masked to allocation when analysing the data. At baseline, and 3, 6, 9, and 12 months, participants in the flash glucose monitoring group wore the professional-mode flash glucose monitoring sensor for 5-14 days before their general practice visit. The sensor recorded interstitial glucose concentrations every 15 min, but the glucose data were not available to the participant until their general practice visit, where the sensor output would be uploaded to a computer by the health professional and discussed. Control group participants wore the sensor at baseline and at 12 months for data analysis only, and had usual care visits every 3 months. The primary outcome was the between-group difference in mean HbA1c at 12 months. Secondary outcomes were the between-group differences in: mean percentage time in target glucose range (4-10 mmol/L), based on ambulatory glucose profile data at 12 months; mean diabetes-specific distress (assessed with the Problem Areas In Diabetes [PAID] scale) at 12 months; and mean HbA1c at 6 months. Analysis was done by intention to treat. This trial is registered at the Australian and New Zealand Clinical Trials Registry, ACTRN12616001372471.
FINDINGS
Between Oct 4, 2016, and Nov 17, 2017, we randomly assigned 299 adults: 149 to flash glucose monitoring and 150 to usual care. At 6 months, HbA1c was lower in the flash glucose monitoring group than in the usual care group (difference -0·5%, 95% CI -0·8% to -0·3%; p=0·0001). However, at 12 months (primary outcome), there was no significant between-group difference in estimated mean HbA1c (8·2% [95% CI 8·0 to 8·4] for flash glucose monitoring vs 8·5% [8·3 to 8·7] for usual care; between-group difference -0·3%, 95% CI -0·5 to 0·01; [66 mmol/mol, 95% CI 64 to 68 vs 69 mmol/mol, 67 to 72; between-group difference -3·0, 95% CI -5·0 to 0·1]; p=0·059). Mean percentage time spent in target glucose range at 12 months was 7·9% (95% CI 2·3 to 13·5) higher in the flash glucose monitoring group than in the usual care group (p=0·0060). Diabetes-specific distress PAID scores were unchanged at 12 months (between-group difference -0·7, 95% CI -3·3 to 1·9; p=0·61). No episodes of severe hypoglycaemia or treatment-related deaths were reported. One participant died during the study from causes unrelated to the intervention (following complications post-myocardial infarction with multiple comorbidities).
INTERPRETATION
Professional-mode flash glucose monitoring in adults with type 2 diabetes in general practice did not improve the primary outcome of HbA1c at 12 months or diabetes-specific distress compared with usual care, but did improve time in target glucose range at 12 months and HbA1c at 6 months. Our findings suggest that professional-mode flash glucose monitoring can be implemented in a pragmatic primary care environment. Although there was no change in HbA1c at 12 months, the improved time in target range might reflect the potential of the technology to support personalised clinical care by providing insights into glycaemic profiles for some people with type 2 diabetes.
FUNDING
National Health and Medical Research Council of Australia, Sanofi Australia, and Abbott Diabetes Care.
中文翻译:

一般模式(GP-OSMOTIC)在成人2型糖尿病患者中每3个月使用专业模式快速血糖监测(GP-OSMOTIC):一项实用,开放标签,12个月的随机对照试验。
背景技术实时(个人)或回顾性(专业模式)的连续葡萄糖监测可以识别每日的葡萄糖概况,以指导2型糖尿病患者的管理决策。我们旨在检查一般模式下每2个月对成人2型糖尿病患者进行专业模式快速血糖监测的效果。方法我们在澳大利亚维多利亚州进行了一项实用的,两臂,开放标签,为期12个月的个体随机对照试验(GP-OSMOTIC),共进行了25次常规治疗。符合条件的参与者是18-80岁的成年人,在过去的一个月中被诊断出至少2年的2型糖尿病和HbA1c至少比其目标高5·5 mmol / mol(0·5%),尽管已处方了至少两种非-胰岛素降糖药,胰岛素或两者兼有(治疗稳定至少4个月)。我们将参与者(1:1)随机分配给使用专业模式快速血糖监测系统或常规临床护理(对照)。所有参与者均在基线时佩戴了快速血糖监测传感器,并在安装传感器后进行了电子随机化(使用4和6的排列组大小,并由临床分层)。不可能对参与者进行掩盖,也不能对临床医生进行分组分配,但是在分析数据时,对研究统计学家进行了掩盖分配。在基线期以及第3、6、9和12个月时,血糖监测组的参与者在进行全科就诊之前,要佩戴专业模式的血糖监测传感器5-14天。传感器每15分钟记录一次组织间葡萄糖浓度,但是直到参与者进行常规访问之前,葡萄糖数据才对参与者可用,在那里,传感器输出将由卫生专业人员上载到计算机上并进行讨论。对照组参与者仅在基线和12个月时才佩戴传感器进行数据分析,并且每3个月进行一次常规护理就诊。主要结局是12个月时平均HbA1c的组间差异。次要结果是组间差异:基于12个月的动态血糖分布数据,目标血糖范围内的平均时间百分比(4-10 mmol / L);12个月时的平均糖尿病特异性困扰(根据糖尿病问题区域[PAID]量表评估);在6个月时的平均HbA1c。分析是按意向进行的。该试验已在澳大利亚和新西兰临床试验注册中心进行了注册,ACTRN12616001372471。结果在2016年10月4日至2017年11月17日之间,我们随机分配了299名成人:149名患者进行血糖监测,而150名患者进行常规护理。在6个月时,快速血糖监测组的HbA1c低于常规护理组(差异-0·5%,95%CI -0·8%至-0·3%; p = 0·0001)。但是,在12个月(主要结局)时,组之间的平均HbA1c估计值之间无显着差异(监测闪血糖的比率为8·2%[95%CI 8·0至8·4],而8·5%[8] [3至8·7]用于常规护理;组间差异-0·3%,95%CI -0·5至0·01; [66 mmol / mol,95%CI 64至68 vs 69 mmol / mol ; 67至72;组间差异-3·0,95%CI -5·0至0·1]; p = 0·059)。闪血糖监测组在12个月目标血糖范围内花费的平均时间百分比比常规护理组高7·9%(95%CI 2·3至13·5)(p = 0·0060)。糖尿病特有的困扰PAID评分在12个月不变(组间差异-0·7,95%CI -3·3至1·9; p = 0·61)。没有严重的低血糖发作或与治疗有关的死亡的报道。一名参与者在研究期间因与干预无关的原因而死亡(继发于心肌梗死后并发多种合并症的并发症)。解释与一般护理相比,一般模式下对成人2型糖尿病成年人进行专业模式快速血糖监测并没有改善HbA1c在12个月时的主要结局或糖尿病特有的困扰,但确实改善了12个月目标血糖范围和HbA1c的时间在6个月。我们的发现表明,可以在务实的初级保健环境中实施专业模式的快速血糖监测。尽管HbA1c在12个月时没有变化,但目标范围内时间的缩短可能反映出该技术通过提供对某些2型糖尿病患者的血糖曲线的洞察力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究委员会,澳大利亚赛诺菲和雅培糖尿病护理。目标范围内时间的缩短可能反映出该技术通过提供洞察某些2型糖尿病患者血糖状况的能力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究理事会,澳大利亚赛诺菲和雅培糖尿病护理。目标范围内时间的缩短可能反映出该技术通过提供洞察某些2型糖尿病患者血糖状况的能力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究理事会,澳大利亚赛诺菲和雅培糖尿病护理。
更新日期:2019-12-18
中文翻译:

一般模式(GP-OSMOTIC)在成人2型糖尿病患者中每3个月使用专业模式快速血糖监测(GP-OSMOTIC):一项实用,开放标签,12个月的随机对照试验。
背景技术实时(个人)或回顾性(专业模式)的连续葡萄糖监测可以识别每日的葡萄糖概况,以指导2型糖尿病患者的管理决策。我们旨在检查一般模式下每2个月对成人2型糖尿病患者进行专业模式快速血糖监测的效果。方法我们在澳大利亚维多利亚州进行了一项实用的,两臂,开放标签,为期12个月的个体随机对照试验(GP-OSMOTIC),共进行了25次常规治疗。符合条件的参与者是18-80岁的成年人,在过去的一个月中被诊断出至少2年的2型糖尿病和HbA1c至少比其目标高5·5 mmol / mol(0·5%),尽管已处方了至少两种非-胰岛素降糖药,胰岛素或两者兼有(治疗稳定至少4个月)。我们将参与者(1:1)随机分配给使用专业模式快速血糖监测系统或常规临床护理(对照)。所有参与者均在基线时佩戴了快速血糖监测传感器,并在安装传感器后进行了电子随机化(使用4和6的排列组大小,并由临床分层)。不可能对参与者进行掩盖,也不能对临床医生进行分组分配,但是在分析数据时,对研究统计学家进行了掩盖分配。在基线期以及第3、6、9和12个月时,血糖监测组的参与者在进行全科就诊之前,要佩戴专业模式的血糖监测传感器5-14天。传感器每15分钟记录一次组织间葡萄糖浓度,但是直到参与者进行常规访问之前,葡萄糖数据才对参与者可用,在那里,传感器输出将由卫生专业人员上载到计算机上并进行讨论。对照组参与者仅在基线和12个月时才佩戴传感器进行数据分析,并且每3个月进行一次常规护理就诊。主要结局是12个月时平均HbA1c的组间差异。次要结果是组间差异:基于12个月的动态血糖分布数据,目标血糖范围内的平均时间百分比(4-10 mmol / L);12个月时的平均糖尿病特异性困扰(根据糖尿病问题区域[PAID]量表评估);在6个月时的平均HbA1c。分析是按意向进行的。该试验已在澳大利亚和新西兰临床试验注册中心进行了注册,ACTRN12616001372471。结果在2016年10月4日至2017年11月17日之间,我们随机分配了299名成人:149名患者进行血糖监测,而150名患者进行常规护理。在6个月时,快速血糖监测组的HbA1c低于常规护理组(差异-0·5%,95%CI -0·8%至-0·3%; p = 0·0001)。但是,在12个月(主要结局)时,组之间的平均HbA1c估计值之间无显着差异(监测闪血糖的比率为8·2%[95%CI 8·0至8·4],而8·5%[8] [3至8·7]用于常规护理;组间差异-0·3%,95%CI -0·5至0·01; [66 mmol / mol,95%CI 64至68 vs 69 mmol / mol ; 67至72;组间差异-3·0,95%CI -5·0至0·1]; p = 0·059)。闪血糖监测组在12个月目标血糖范围内花费的平均时间百分比比常规护理组高7·9%(95%CI 2·3至13·5)(p = 0·0060)。糖尿病特有的困扰PAID评分在12个月不变(组间差异-0·7,95%CI -3·3至1·9; p = 0·61)。没有严重的低血糖发作或与治疗有关的死亡的报道。一名参与者在研究期间因与干预无关的原因而死亡(继发于心肌梗死后并发多种合并症的并发症)。解释与一般护理相比,一般模式下对成人2型糖尿病成年人进行专业模式快速血糖监测并没有改善HbA1c在12个月时的主要结局或糖尿病特有的困扰,但确实改善了12个月目标血糖范围和HbA1c的时间在6个月。我们的发现表明,可以在务实的初级保健环境中实施专业模式的快速血糖监测。尽管HbA1c在12个月时没有变化,但目标范围内时间的缩短可能反映出该技术通过提供对某些2型糖尿病患者的血糖曲线的洞察力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究委员会,澳大利亚赛诺菲和雅培糖尿病护理。目标范围内时间的缩短可能反映出该技术通过提供洞察某些2型糖尿病患者血糖状况的能力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究理事会,澳大利亚赛诺菲和雅培糖尿病护理。目标范围内时间的缩短可能反映出该技术通过提供洞察某些2型糖尿病患者血糖状况的能力来支持个性化临床护理的潜力。资助澳大利亚国家卫生和医学研究理事会,澳大利亚赛诺菲和雅培糖尿病护理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号