当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Therapeutically targeting head and neck squamous cell carcinoma through synergistic inhibition of LSD1 and JMJD3 by TCP and GSK-J1.
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-12-18 , DOI: 10.1038/s41416-019-0680-6 Wei Zhang 1 , Jie Cheng 1 , Pengfei Diao 1 , Dongmiao Wang 2 , Wei Zhang 3 , Hongbing Jiang 2 , Yanling Wang 1
British Journal of Cancer ( IF 6.4 ) Pub Date : 2019-12-18 , DOI: 10.1038/s41416-019-0680-6 Wei Zhang 1 , Jie Cheng 1 , Pengfei Diao 1 , Dongmiao Wang 2 , Wei Zhang 3 , Hongbing Jiang 2 , Yanling Wang 1
Affiliation
|
BACKGROUND
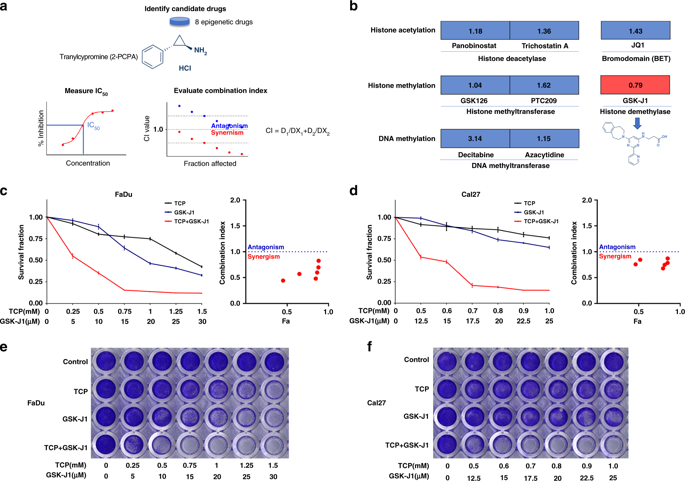
The histone demethylase LSD1 is a key mediator driving tumorigenesis, which holds potential as a promising therapeutic target. However, treatment with LSD1 inhibitors alone failed to result in complete cancer regression.
METHODS
The synergistic effects of TCP (a LSD1 inhibitor) and GSK-J1 (a JMJD3 inhibitor) against HNSCC were determined in vitro and in preclinical animal models. Genes modulated by chemical agents or siRNAs in HNSCC cells were identified by RNA-seq and further functionally interrogated by bioinformatics approach. Integrative siRNA-mediated gene knockdown, rescue experiment and ChIP-qPCR assays were utilised to characterise the mediators underlying the therapeutic effects conferred by TCP and GSK-J1.
RESULTS
Treatment with TCP and GSK-J1 impaired cell proliferation, induced apoptosis and senescence in vitro, which were largely recapitulated by simultaneous LSD1 and JMJD3 knockdown. Combinational treatment inhibited tumour growth and progression in vivo. Differentially expressed genes modulated by TCP and GSK-J1 were significantly enriched in cell proliferation, apoptosis and cancer-related pathways. SPP1 was identified as the mediator of synergy underlying the pro-apoptosis effects conferred by TCP and GSK-J1. Co-upregulation of LSD1 and JMJD3 associated with worse prognosis in patients with HNSCC.
CONCLUSIONS
Our findings revealed a novel therapeutic strategy of simultaneous LSD1 and JMJD3 inhibition against HNSCC.
中文翻译:

通过 TCP 和 GSK-J1 协同抑制 LSD1 和 JMJD3 治疗头颈部鳞状细胞癌。
背景技术组蛋白去甲基化酶 LSD1 是驱动肿瘤发生的关键介质,具有作为有前途的治疗靶点的潜力。然而,单独使用 LSD1 抑制剂治疗未能使癌症完全消退。方法 在体外和临床前动物模型中确定 TCP(一种 LSD1 抑制剂)和 GSK-J1(一种 JMJD3 抑制剂)对 HNSCC 的协同作用。通过 RNA-seq 鉴定 HNSCC 细胞中由化学试剂或 siRNA 调节的基因,并通过生物信息学方法进一步进行功能查询。利用整合的 siRNA 介导的基因敲低、拯救实验和 ChIP-qPCR 测定来表征 TCP 和 GSK-J1 赋予的治疗效果的潜在介质。结果用 TCP 和 GSK-J1 处理会损害细胞增殖,在体外诱导细胞凋亡和衰老,同时 LSD1 和 JMJD3 击倒在很大程度上概括了这一点。联合治疗抑制体内肿瘤生长和进展。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。
更新日期:2019-12-18
中文翻译:

通过 TCP 和 GSK-J1 协同抑制 LSD1 和 JMJD3 治疗头颈部鳞状细胞癌。
背景技术组蛋白去甲基化酶 LSD1 是驱动肿瘤发生的关键介质,具有作为有前途的治疗靶点的潜力。然而,单独使用 LSD1 抑制剂治疗未能使癌症完全消退。方法 在体外和临床前动物模型中确定 TCP(一种 LSD1 抑制剂)和 GSK-J1(一种 JMJD3 抑制剂)对 HNSCC 的协同作用。通过 RNA-seq 鉴定 HNSCC 细胞中由化学试剂或 siRNA 调节的基因,并通过生物信息学方法进一步进行功能查询。利用整合的 siRNA 介导的基因敲低、拯救实验和 ChIP-qPCR 测定来表征 TCP 和 GSK-J1 赋予的治疗效果的潜在介质。结果用 TCP 和 GSK-J1 处理会损害细胞增殖,在体外诱导细胞凋亡和衰老,同时 LSD1 和 JMJD3 击倒在很大程度上概括了这一点。联合治疗抑制体内肿瘤生长和进展。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。由 TCP 和 GSK-J1 调节的差异表达基因在细胞增殖、凋亡和癌症相关通路中显着富集。SPP1 被确定为 TCP 和 GSK-J1 赋予的促细胞凋亡作用的协同作用的中介。LSD1 和 JMJD3 的共同上调与 HNSCC 患者较差的预后相关。结论 我们的研究结果揭示了一种针对 HNSCC 同时抑制 LSD1 和 JMJD3 的新型治疗策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号