当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clinically Relevant OATP2B1 Inhibitors in Marketed Drug Space.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.molpharmaceut.9b00897 Melissa S Unger 1, 2 , Jennypher Mudunuru 3 , Matthias Schwab 4, 5 , Carsten Hopf 2 , Gerard Drewes 1 , Anne T Nies 4 , Maciej J Zamek-Gliszczynski 3 , Friedrich B M Reinhard 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-01-02 , DOI: 10.1021/acs.molpharmaceut.9b00897 Melissa S Unger 1, 2 , Jennypher Mudunuru 3 , Matthias Schwab 4, 5 , Carsten Hopf 2 , Gerard Drewes 1 , Anne T Nies 4 , Maciej J Zamek-Gliszczynski 3 , Friedrich B M Reinhard 1
Affiliation
|
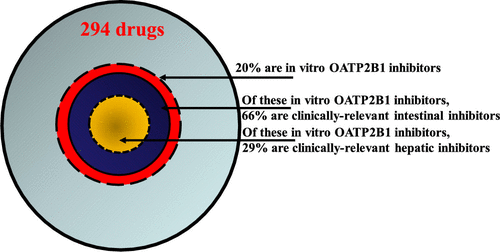
OATP2B1 is an intestinal and hepatic drug uptake transporter. Intestinal OATP2B1 has been elucidated as the mechanism of unexpected clinical drug-drug interactions (DDIs), where drug exposure was unexpectedly decreased with unchanged half-life. Hepatic OATP2B1 may be an understudied clinical DDI mechanism. The aim of the present work was to understand the prevalence of clinically relevant intestinal and hepatic OATP2B1 inhibitors in marketed drug space. HEK293 cells stably overexpressing human OATP2B1 or vector control were generated and cultured for 72 h in a 96-well format. OATP2B1-mediated uptake of dibromofluorescein (DBF) was found to be optimal at 10 μM concentration and 30 min incubation time. A total of 294 drugs (top 300 marketed drugs, excluding biologics and restricted drugs, supplemented with ∼100 small-molecule drugs) were screened for OATP2B1 inhibition at 10 μM. Drugs demonstrating ≥50% inhibition in this screen were advanced for IC50 determination, which was extrapolated to clinical intestinal and hepatic OATP2B1 inhibition as per 2017 FDA DDI guidance. Of the 294 drugs screened, 67 elicited ≥50% inhibition of OATP2B1-mediated DBF uptake at 10 μM screening concentration. For the 67 drugs flagged in the single-concentration inhibition screen, upon evaluation of a full concentration range, IC50 values could be determined for 58 drugs. OATP2B1 IC50 values established for these 58 drugs were extrapolated as potentially clinically relevant at the intestinal level for 38 orally administered drugs (Igut/IC50 ≥ 10), and 17 were flagged as potential clinical inhibitors of hepatic OATP2B1 uptake (1 + Iin,max,u/IC50 ≥ 1.1). This analysis of 294 drugs demonstrated prevalence of clinically relevant intestinal and hepatic OATP2B1 inhibitors to be 13 and 6%, respectively. As OATP2B1-inhibitor drugs are not exceedingly rare, these results suggest that clinical OATP2B1 DDIs have been rarely observed because OATP2B1 is uncommonly the predominant determinant of drug disposition.
中文翻译:

市售药物领域中与临床相关的OATP2B1抑制剂。
OATP2B1是肠道和肝脏吸收药物的转运蛋白。肠道OATP2B1已被阐明为意料之外的临床药物相互作用(DDI)的机制,其中药物暴露意外降低且半衰期不变。肝OATP2B1可能是尚未被研究的临床DDI机制。本工作的目的是了解上市的药物领域中临床相关的肠和肝OATP2B1抑制剂的普遍性。产生稳定过表达人OATP2B1或载体对照的HEK293细胞,并以96孔形式培养72小时。发现OATP2B1介导的二溴荧光素(DBF)摄取在10μM浓度和30分钟孵育时间下是最佳的。共有294种药物(市场上排名前300位的药物,不包括生物制剂和限制性药物,在补充了约100种小分子药物的条件下,筛选了10μM的OATP2B1抑制作用。在该筛选中显示出≥50%抑制作用的药物已提前用于IC50测定,根据2017 FDA DDI指南外推至临床肠道和肝脏OATP2B1抑制作用。在筛选的294种药物中,有67种在10μM的筛选浓度下引起OATP2B1介导的DBF摄取抑制率≥50%。对于单浓度抑制筛选中标记的67种药物,在评估整个浓度范围后,可以确定58种药物的IC50值。对于这38种口服药物(Igut / IC50≥10),推断这58种药物建立的OATP2B1 IC50值在肠道水平上具有潜在的临床相关性,其中17种被标记为潜在的肝OATP2B1摄取临床抑制剂(1 + Iin,max, u / IC50≥1.1)。对294种药物的分析表明,临床上相关的肠和肝OATP2B1抑制剂的患病率分别为13和6%。由于OATP2B1抑制剂药物并非十分罕见,因此这些结果表明临床OATP2B1 DDI很少见,因为OATP2B1通常不是药物处置的主要决定因素。
更新日期:2020-01-02
中文翻译:

市售药物领域中与临床相关的OATP2B1抑制剂。
OATP2B1是肠道和肝脏吸收药物的转运蛋白。肠道OATP2B1已被阐明为意料之外的临床药物相互作用(DDI)的机制,其中药物暴露意外降低且半衰期不变。肝OATP2B1可能是尚未被研究的临床DDI机制。本工作的目的是了解上市的药物领域中临床相关的肠和肝OATP2B1抑制剂的普遍性。产生稳定过表达人OATP2B1或载体对照的HEK293细胞,并以96孔形式培养72小时。发现OATP2B1介导的二溴荧光素(DBF)摄取在10μM浓度和30分钟孵育时间下是最佳的。共有294种药物(市场上排名前300位的药物,不包括生物制剂和限制性药物,在补充了约100种小分子药物的条件下,筛选了10μM的OATP2B1抑制作用。在该筛选中显示出≥50%抑制作用的药物已提前用于IC50测定,根据2017 FDA DDI指南外推至临床肠道和肝脏OATP2B1抑制作用。在筛选的294种药物中,有67种在10μM的筛选浓度下引起OATP2B1介导的DBF摄取抑制率≥50%。对于单浓度抑制筛选中标记的67种药物,在评估整个浓度范围后,可以确定58种药物的IC50值。对于这38种口服药物(Igut / IC50≥10),推断这58种药物建立的OATP2B1 IC50值在肠道水平上具有潜在的临床相关性,其中17种被标记为潜在的肝OATP2B1摄取临床抑制剂(1 + Iin,max, u / IC50≥1.1)。对294种药物的分析表明,临床上相关的肠和肝OATP2B1抑制剂的患病率分别为13和6%。由于OATP2B1抑制剂药物并非十分罕见,因此这些结果表明临床OATP2B1 DDI很少见,因为OATP2B1通常不是药物处置的主要决定因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号