Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.jfluchem.2019.109436 Yongsheng Niu , Lixin Sun , Congwei Hu , Jinhao Zhou , Qiang Dou , Qingnuan Li
|
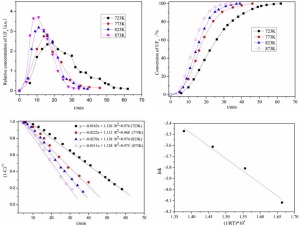
The fluorination of UF4 by NF3 at different temperatures was investigated using in-line Fourier transform infrared (FTIR) spectroscopy. The results indicated that the fluorination between NF3 and UF4 occurred only when the temperature was above 723 K, and the temperature had a significant effect on the average reaction rate and the utilization rate of NF3. The in-line FTIR spectrum analysis and thermodynamic calculation indicated that UF4 was converted to UF6 with the following reaction mechanism: 2/3NF3(g) + UF4(s) = UF6(g) + 1/3N2(g). The conversion rate of UF4 to UF6 increased from 1.53 to 2.68 gU•h-1 and the utilization rate of NF3 increased from 10.5% to 22.5% when the reaction temperature increased from 723 K to 873 K. The calculated rate constant increased from 1.6 × 10-2 to 3.1 × 10-2 min-1 in the range of 723 K to 873 K, and the activation energy of the reaction was 22.56 kJ/mol.
中文翻译:

三氟化氮对四氟化铀的氟化反应研究
使用在线傅立叶变换红外(FTIR)光谱研究了在不同温度下NF 3对UF 4的氟化作用。结果表明,仅当温度高于723 K时,NF 3和UF 4之间才发生氟化反应,该温度对NF 3的平均反应速率和利用率有显着影响。在线FTIR光谱分析和热力学计算表明,UF 4通过以下反应机理转化为UF 6:2 / 3NF 3(g)+ UF 4(s)= UF 6(g)+ 1 / 3N 2(G)。当反应温度从723 K增加到873 K时,UF 4转化为UF 6的转化率从1.53增加到2.68 gU•h -1,NF 3的利用率从10.5%增加到22.5%。计算的速率常数增加在723 K至873 K的范围内,从1.6×10 -2到3.1×10 -2 min -1,反应的活化能为22.56 kJ / mol。









































 京公网安备 11010802027423号
京公网安备 11010802027423号