Journal of Catalysis ( IF 6.5 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.jcat.2019.11.018 Hongye Qin , Biao Zhang , Yaping Pan , Xixi Wang , Lechen Diao , Jiao Chen , Jinli Wu , Enzuo Liu , Junwei Sha , Liying Ma , Naiqin Zhao
|
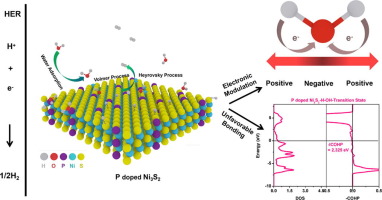
Developing efficient and low-cost electrocatalysts for hydrogen evolution reaction (HER) is important in clean energy systems. Non-noble transition metals are the most promising candidates for the replacement of conventional Pt group catalysts of HER. However, most non-noble metals show poor HER activity due to the intrinsic electronic structures. Herein, P atoms doped Ni3S2 (P-doped Ni3S2) nanosheets array grown on Ni foam has been successfully synthesized and further applied as the efficient electrocatalysts for HER in alkaline media. P-doped Ni3S2 shows higher catalytic activity for HER compared with pristine Ni3S2, which affords the current densities of 10 mA cm−2 at an overpotential of 139 mV and long-term stability over 110 h. Density functional theory (DFT) calculations reveal that the introduction of P atoms modify the electronic structure of Ni3S2, enhance the electrical conductivity, optimize the HER Gibbs free-energy (ΔGH*) and the change of water adsorption energy (ΔGH2O*), and reduce the barrier of water dissociation optimize the HER Gibbs free-energy (ΔGH*), change water adsorption energy (ΔGH2O*) and reduce the barrier of water dissociation. Remarkably, energy integral of a crystal orbital Hamilton population (ICOHP) gives access to the contribution of an atom or a chemical bond to the distribution of one-particle bonding within the transistion state of water dissociation and reveals the reduced essence of water dissociation barrier.
中文翻译:

通过P诱导电子调制加速Ni 3 S 2纳米片上的水离解动力学
开发用于氢气析出反应(HER)的高效且低成本的电催化剂在清洁能源系统中很重要。非贵金属过渡金属是替代HER的传统Pt基催化剂的最有希望的候选者。然而,由于固有的电子结构,大多数非贵金属显示出较差的HER活性。在此,已经成功地合成了在Ni泡沫上生长的P原子掺杂的Ni 3 S 2(P掺杂的Ni 3 S 2)纳米片阵列,并将其进一步用作HER在碱性介质中的有效电催化剂。与原始Ni 3 S 2相比,掺P的Ni 3 S 2对HER的催化活性更高。,在139 mV的超电势下提供10 mA cm -2的电流密度,并在110 h内具有长期稳定性。密度泛函理论(DFT)计算表明,引入P原子可改变Ni 3 S 2的电子结构,增强电导率,优化HER Gibbs自由能(ΔG H *)和吸水能的变化( Δ ģ H2O *),并且减少水的离解优化的屏障的HER吉布斯自由能(Δ G ^ H *),改变水的吸附能(Δ ģ H2O *),并减少了水离解的障碍。值得注意的是,晶体轨道汉密尔顿族(ICOHP)的能量积分使原子或化学键对水解离的跃迁状态内的单粒子键合的分布具有可及性,并揭示了水离解势垒的本质降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号