当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Charge-reversal nanocarriers: An emerging paradigm for smart cancer nanomedicine.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.jconrel.2019.12.024 Mingzhen Zhang 1 , Xiaoxiao Chen 2 , Chao Li 3 , Xian Shen 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.jconrel.2019.12.024 Mingzhen Zhang 1 , Xiaoxiao Chen 2 , Chao Li 3 , Xian Shen 1
Affiliation
|
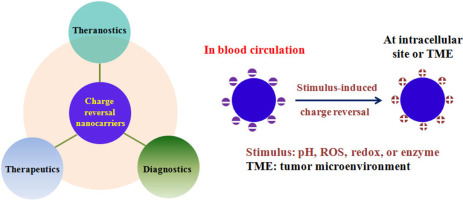
The surface charge of nanoparticles (NPs) plays an essential role in determining their biological properties both in vitro and in vivo. In view of the complex features associated with the biological or physiological microenvironment of solid tumors, such as electrostatic interactions between NPs and serum components, cellular membrane, or intracellular organelles, drug-loaded NPs (or nanocarriers) should intelligently accommodate such unique extra- or intracellular microenvironment in order to achieve maximum therapeutic and/or diagnostic efficacy. To that end, the surface charge of nanocarriers needs to be readily converted at the target site by means of charge reversal, i.e., conversion from anionic to cationic, or vice versa, depending on specific microenvironment. In such a manner, the payloads could be efficiently released at the desired tumor site. This review discusses 1) the physicochemical aspects related to long-circulating nanocarriers for systemic applications; 2) the recent progress in charge-reversal nanocarriers, which are loaded with drugs, nucleic acids, proteins or imaging agents and triggered by various biological signals (i.e., pH, redox, ROS or enzyme) associated with tumor microenvironment, with an emphasis on those induced by acidic tumoral pH; and 3) the perspectives of charge-reversal nanocarriers regarding thorough investigations on how the chemical structure of charge-reversal moiety temporally affects the responsiveness of the resulting nanocarriers toward the rational design of precision cancer nanomedicine.
中文翻译:

电荷逆转纳米载体:智能癌症纳米医学的新兴范例。
纳米粒子(NPs)的表面电荷在确定其体内和体外生物学特性中起着至关重要的作用。考虑到与实体瘤的生物学或生理学微环境相关的复杂特征,例如NP与血清成分,细胞膜或细胞内细胞器之间的静电相互作用,载有药物的NP(或纳米载体)应智能地适应这种独特的外部或外部环境。细胞内微环境以达到最大的治疗和/或诊断功效。为此,取决于特定的微环境,纳米载体的表面电荷需要通过电荷逆转而容易地在靶位处转化,即从阴离子到阳离子的转化,反之亦然。这样,有效载荷可以在所需的肿瘤部位有效释放。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。蛋白质或显像剂,并由与肿瘤微环境有关的各种生物信号(例如,pH,氧化还原,ROS或酶)触发,重点是酸性肿瘤pH诱导的信号;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。蛋白质或显像剂,并由与肿瘤微环境有关的各种生物信号(例如,pH,氧化还原,ROS或酶)触发,重点是酸性肿瘤pH诱导的信号;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。
更新日期:2019-12-17
中文翻译:

电荷逆转纳米载体:智能癌症纳米医学的新兴范例。
纳米粒子(NPs)的表面电荷在确定其体内和体外生物学特性中起着至关重要的作用。考虑到与实体瘤的生物学或生理学微环境相关的复杂特征,例如NP与血清成分,细胞膜或细胞内细胞器之间的静电相互作用,载有药物的NP(或纳米载体)应智能地适应这种独特的外部或外部环境。细胞内微环境以达到最大的治疗和/或诊断功效。为此,取决于特定的微环境,纳米载体的表面电荷需要通过电荷逆转而容易地在靶位处转化,即从阴离子到阳离子的转化,反之亦然。这样,有效载荷可以在所需的肿瘤部位有效释放。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。这篇综述讨论了1)与用于系统应用的长循环纳米载体有关的物理化学方面;2)载有药物,核酸,蛋白质或显像剂并由与肿瘤微环境有关的各种生物信号(即pH,氧化还原,ROS或酶)触发的电荷可逆纳米载体的最新进展,重点是由酸性肿瘤pH诱导的那些;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。蛋白质或显像剂,并由与肿瘤微环境有关的各种生物信号(例如,pH,氧化还原,ROS或酶)触发,重点是酸性肿瘤pH诱导的信号;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。蛋白质或显像剂,并由与肿瘤微环境有关的各种生物信号(例如,pH,氧化还原,ROS或酶)触发,重点是酸性肿瘤pH诱导的信号;3)关于彻底研究电荷可逆部分化学结构如何暂时影响所得纳米载体对精确癌症纳米药物合理设计的反应性的彻底研究的观点。









































 京公网安备 11010802027423号
京公网安备 11010802027423号