当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new tandem synthesis of bis(β,β'-dialkoxy carbonyl) compounds by oxidative cleavage of aziridines under metal-free conditions.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02451d Satyajit Samanta 1 , Sougata Santra , Rana Chatterjee , Adinath Majee
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02451d Satyajit Samanta 1 , Sougata Santra , Rana Chatterjee , Adinath Majee
Affiliation
|
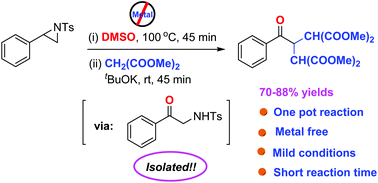
An efficient and new approach has been developed to synthesize bis(β,β'-dialkoxy carbonyl) derivatives through the reaction between N-tosylaziridines and malonate esters under ambient air using tBuOK in DMSO solvent. A plausible reaction pathway has been predicted. Control experiments suggested that the reactions proceed through the formation of α-aminoketones. This reaction offers a broad substrate scope, metal-free synthesis, excellent regioselectivity, easily accessible reactants, and simple operation. A gram-scale synthesis demonstrates the potential applications of the present method.
中文翻译:

在无金属条件下通过氮丙啶的氧化裂解串联双(β,β'-二烷氧基羰基)化合物的新方法。
通过在DMSO溶剂中使用tBuOK在环境空气下,N-甲苯磺酰氮丙啶与丙二酸酯之间的反应,开发了一种有效的新方法来合成双(β,β'-二烷氧基羰基)衍生物。已经预测到可能的反应途径。对照实验表明,反应是通过形成α-氨基酮来进行的。该反应提供了广泛的底物范围,无金属合成,出色的区域选择性,易于获得的反应物以及简单的操作。克级合成证明了本方法的潜在应用。
更新日期:2020-01-02
中文翻译:

在无金属条件下通过氮丙啶的氧化裂解串联双(β,β'-二烷氧基羰基)化合物的新方法。
通过在DMSO溶剂中使用tBuOK在环境空气下,N-甲苯磺酰氮丙啶与丙二酸酯之间的反应,开发了一种有效的新方法来合成双(β,β'-二烷氧基羰基)衍生物。已经预测到可能的反应途径。对照实验表明,反应是通过形成α-氨基酮来进行的。该反应提供了广泛的底物范围,无金属合成,出色的区域选择性,易于获得的反应物以及简单的操作。克级合成证明了本方法的潜在应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号