当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Michael additions on α-aminoacrylates as the key step to an l-Oic analogue bearing a quaternary stereocenter.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02084e Federico Maria Cecchinelli 1 , Giuseppe Celentano 1 , Alessandra Puglisi 2 , Nicoletta Gaggero 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02084e Federico Maria Cecchinelli 1 , Giuseppe Celentano 1 , Alessandra Puglisi 2 , Nicoletta Gaggero 1
Affiliation
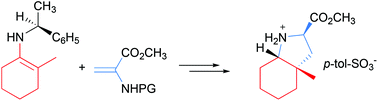
|
A novel, highly stereoselective route for pharmaceutically relevant octahydroindole-2-carboxylates bearing a quaternary stereocenter has been developed. The key chiral intermediates 3 have been prepared in good yields and enantiomeric excesses up to 98%. A broad substrate range has been tolerated under the reaction conditions.
中文翻译:

在α-氨基丙烯酸酯上的立体选择性迈克尔加成是带有四元立体中心的l-Oic类似物的关键步骤。
已开发出具有季立体中心的药学上相关的八氢吲哚-2-羧酸酯的新颖的,高度立体选择性的途径。关键的手性中间体3的制备得率很高,对映体过量高达98%。在反应条件下可以耐受较宽的底物范围。
更新日期:2020-02-10
中文翻译:

在α-氨基丙烯酸酯上的立体选择性迈克尔加成是带有四元立体中心的l-Oic类似物的关键步骤。
已开发出具有季立体中心的药学上相关的八氢吲哚-2-羧酸酯的新颖的,高度立体选择性的途径。关键的手性中间体3的制备得率很高,对映体过量高达98%。在反应条件下可以耐受较宽的底物范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号