当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvometallurgical process for extraction of copper from chalcopyrite and other sulfidic ore minerals
Green Chemistry ( IF 9.3 ) Pub Date : 2019/12/13 , DOI: 10.1039/c9gc02983d Xiaohua Li 1, 2, 3, 4 , Wouter Monnens 1, 4, 5, 6 , Zheng Li 1, 2, 3, 4 , Jan Fransaer 1, 4, 5, 6 , Koen Binnemans 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2019/12/13 , DOI: 10.1039/c9gc02983d Xiaohua Li 1, 2, 3, 4 , Wouter Monnens 1, 4, 5, 6 , Zheng Li 1, 2, 3, 4 , Jan Fransaer 1, 4, 5, 6 , Koen Binnemans 1, 2, 3, 4
Affiliation
|
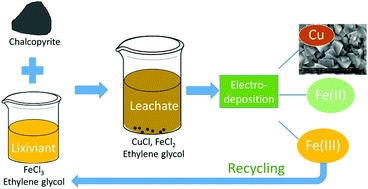
Extraction of copper from sufidic ores, either by pyrometallurgy or hydrometallurgy, has various limitations. In this study, a solvometallurgical process for the extraction of copper from sulfidic ore minerals (chalcopyrite, bornite, chalcocite and digenite) was developed by using an organic lixiviant (FeCl3 as oxidizing agent and ethylene glycol (EG) as organic solvent). All the studied copper sulfide minerals could be leached efficiently with a FeCl3–EG solution. Other lixiviant systems, namely CuCl2–EG, FeCl3–ethanol and FeCl3–propylene glycol could also extract copper, but they did not perform as well as the FeCl3–EG solutions. The mechanistic study of chalcopyrite leaching in FeCl3–EG solutions confirmed that the leaching products of chalcopyrite were FeCl2, CuCl and solid elemental sulfur, where the Fe(II) and Cu(I) were quantified by UV-Vis absorption spectroscopy and solid sulfur was identified by powder XRD. A kinetic study showed that the leaching process was a surface chemical controlled process and the apparent activation energy was calculated to be 60.1 kJ mol−1. Subsequently, electrodeposition of copper from the pregnant leachate was investigated, and SEM-EDX analysis showed that uniform cubic crystalline deposits of pure copper were produced. Meanwhile, the Fe(III) was regenerated by oxidizing Fe(II) at the anode, with a Morgane membrane in between two electrode compartments to prevent the transfer of Fe(III) to the cathode. Finally, a closed-loop solvometallurgical process was designed with three operational steps: leaching, electrodeposition and removal of Fe(II). The regeneration of the FeCl3–EG solution and the use of EG contribute to the sustainability and the greenness of the process.
中文翻译:

溶剂冶金法从黄铜矿和其他硫化矿矿物中提取铜
通过火法冶金或湿法冶金从次生矿石中提取铜具有多种局限性。在这项研究中,通过使用有机浸滤剂(FeCl 3作为氧化剂,乙二醇(EG)作为有机溶剂),开发了一种溶剂冶金工艺,用于从硫化矿石矿物(黄铜矿,褐铁矿,黄铜矿和方岩)中提取铜。用FeCl 3 -EG溶液可以有效地浸出所有已研究的硫化铜矿物。其他浸滤系统,例如CuCl 2 -EG,FeCl 3-乙醇和FeCl 3-丙二醇也可以萃取铜,但性能不如FeCl 3。–EG解决方案。在FeCl 3 -EG溶液中黄铜矿浸出的机理研究证实,黄铜矿的浸出产物为FeCl 2,CuCl和固体元素硫,其中的Fe(II)和Cu(I)通过UV-Vis吸收光谱和固体进行定量。通过粉末XRD鉴定了硫。动力学研究表明,浸出过程是表面化学控制的过程,其表观活化能经计算为60.1 kJ mol -1。随后,研究了从浸出液中铜的电沉积,并且SEM-EDX分析表明,生成了均匀的纯铜立方晶沉积物。同时,Fe(III通过在阳极氧化Fe(II)来再生Fe ),在两个电极隔室之间装有Morgane膜,以防止Fe(III)转移到阴极。最后,设计了一个闭环溶剂冶金工艺,该工艺包括三个操作步骤:浸出,电沉积和去除Fe(II)。FeCl 3 -EG溶液的再生和EG的使用有助于该过程的可持续性和绿色化。
更新日期:2020-02-10
中文翻译:

溶剂冶金法从黄铜矿和其他硫化矿矿物中提取铜
通过火法冶金或湿法冶金从次生矿石中提取铜具有多种局限性。在这项研究中,通过使用有机浸滤剂(FeCl 3作为氧化剂,乙二醇(EG)作为有机溶剂),开发了一种溶剂冶金工艺,用于从硫化矿石矿物(黄铜矿,褐铁矿,黄铜矿和方岩)中提取铜。用FeCl 3 -EG溶液可以有效地浸出所有已研究的硫化铜矿物。其他浸滤系统,例如CuCl 2 -EG,FeCl 3-乙醇和FeCl 3-丙二醇也可以萃取铜,但性能不如FeCl 3。–EG解决方案。在FeCl 3 -EG溶液中黄铜矿浸出的机理研究证实,黄铜矿的浸出产物为FeCl 2,CuCl和固体元素硫,其中的Fe(II)和Cu(I)通过UV-Vis吸收光谱和固体进行定量。通过粉末XRD鉴定了硫。动力学研究表明,浸出过程是表面化学控制的过程,其表观活化能经计算为60.1 kJ mol -1。随后,研究了从浸出液中铜的电沉积,并且SEM-EDX分析表明,生成了均匀的纯铜立方晶沉积物。同时,Fe(III通过在阳极氧化Fe(II)来再生Fe ),在两个电极隔室之间装有Morgane膜,以防止Fe(III)转移到阴极。最后,设计了一个闭环溶剂冶金工艺,该工艺包括三个操作步骤:浸出,电沉积和去除Fe(II)。FeCl 3 -EG溶液的再生和EG的使用有助于该过程的可持续性和绿色化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号