当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of catalysts for ammonia synthesis based on metal phthalocyanine materials
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9cy02326g Natalia Morlanés 1, 2, 3, 4 , Walid Almaksoud 1, 2, 3, 4 , Rohit K. Rai 1, 2, 3, 4 , Samy Ould-Chikh 1, 2, 3, 4 , Mohammed M. Ali 4, 5, 6, 7 , Balamurugan Vidjayacoumar 4, 5, 6, 7 , Bedour E. Al-Sabban 4, 5, 6, 7 , Khalid Albahily 4, 5, 6, 7 , Jean-Marie Basset 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9cy02326g Natalia Morlanés 1, 2, 3, 4 , Walid Almaksoud 1, 2, 3, 4 , Rohit K. Rai 1, 2, 3, 4 , Samy Ould-Chikh 1, 2, 3, 4 , Mohammed M. Ali 4, 5, 6, 7 , Balamurugan Vidjayacoumar 4, 5, 6, 7 , Bedour E. Al-Sabban 4, 5, 6, 7 , Khalid Albahily 4, 5, 6, 7 , Jean-Marie Basset 1, 2, 3, 4
Affiliation
|
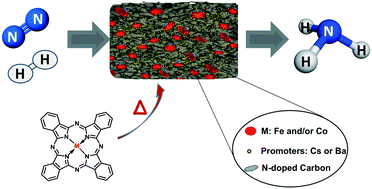
Highly efficient and very stable iron and/or cobalt-based catalysts for the ammonia synthesis reaction were synthesized by one-step pyrolysis of metal phthalocyanine precursors. The presence of alkaline earth or alkali metals is found to be essential for accelerating the reaction rate for the ammonia synthesis process. When promoted by alkali metals, the catalysts show a 3-fold increase in their catalytic performance (at 400 °C and 0.1–7 MPa) compared to a commercial benchmark iron-based catalyst, widely used for the Haber–Bosch process. TEM images reveal the local structure of the catalysts obtained upon pyrolysis of the metal phthalocyanine precursor, with metal nanoparticles (5–50 nm) confined in a nitrogen-doped carbon mesoporous matrix, where the alkali metal promoters are located on the top of the iron nanoparticles but also on the carbon support. Finally, kinetic analysis shows a lower activation energy for the Fe phthalocyanine-derived catalyst (42 kJ mol−1) versus 70 kJ mol−1 reported for the iron-benchmark catalyst. Furthermore, this kinetic analysis suggests that the rate-determining step shifts from nitrogen activation to NHx formation, which only few catalysts have achieved.
中文翻译:

基于金属酞菁原料的氨合成催化剂的开发
通过金属酞菁前体的一步热解合成了用于氨合成反应的高效,非常稳定的铁和/或钴基催化剂。发现碱土或碱金属的存在对于加速氨合成过程的反应速率是必不可少的。当被碱金属促进时,与广泛用于Haber-Bosch工艺的商业基准铁基催化剂相比,该催化剂的催化性能(在400°C和0.1-7 MPa下)显示出3倍的增长。TEM图像揭示了金属酞菁前体热解后获得的催化剂的局部结构,其中金属纳米颗粒(5–50 nm)被限制在氮掺杂的碳介孔基质中,其中碱金属促进剂位于铁纳米颗粒的顶部,但也位于碳载体上。最后,动力学分析表明,Fe酞菁衍生催化剂的活化能较低(42 kJ mol-1)与铁基准催化剂报道的70 kJ mol -1相比。此外,该动力学分析表明,确定速率的步骤从氮活化转变为形成NH x,这仅能实现很少的催化剂。
更新日期:2020-02-13
中文翻译:

基于金属酞菁原料的氨合成催化剂的开发
通过金属酞菁前体的一步热解合成了用于氨合成反应的高效,非常稳定的铁和/或钴基催化剂。发现碱土或碱金属的存在对于加速氨合成过程的反应速率是必不可少的。当被碱金属促进时,与广泛用于Haber-Bosch工艺的商业基准铁基催化剂相比,该催化剂的催化性能(在400°C和0.1-7 MPa下)显示出3倍的增长。TEM图像揭示了金属酞菁前体热解后获得的催化剂的局部结构,其中金属纳米颗粒(5–50 nm)被限制在氮掺杂的碳介孔基质中,其中碱金属促进剂位于铁纳米颗粒的顶部,但也位于碳载体上。最后,动力学分析表明,Fe酞菁衍生催化剂的活化能较低(42 kJ mol-1)与铁基准催化剂报道的70 kJ mol -1相比。此外,该动力学分析表明,确定速率的步骤从氮活化转变为形成NH x,这仅能实现很少的催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号