Catalysis Today ( IF 5.2 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.cattod.2019.12.022 Do-Hyung Kim , Eunae Lee , Chanho Pak
|
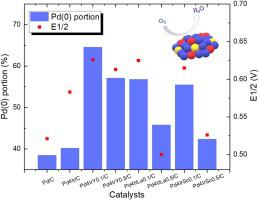
To enhance the catalytic activity of Pd toward the oxygen reduction reaction (ORR), transition metals are alloyed with Pd. This changes the electronic structure of Pd because alloyed transition metal induces a lattice mismatch and ligand effects. Rare-earth elements have a much lower electronegativity, and can therefore donate electrons easily and fill the antibonding orbital of Pd. Therefore, rare-earth elements are effective in increasing the ORR activity by making Pd more metallic. Moreover, alloying Ir with noble metals enhances the ORR activity via the strain effect in an acid medium. In this study, rare-earth elements such as Y, Sc, and La were alloyed with Pd and Ir using a polyol process. As the rare-earth elements have larger atomic sizes and much lower reduction potentials than those of Pd, the amount that can be incorporated in Pd based alloy catalysts with rare-earth elements via the polyol process is limited. The addition of rare-earth elements increased the amount of metallic Pd; however, when added in excess, most of the rare-earth elements on the surface were located in the grain boundary in the form of oxides. When the composition ratio of Pd, Ir, and rare-earth elements was 4:1:0.1, Pd–Ir–Y, Pd–Ir–La and Pd–Ir–Sc alloy catalysts had higher metallic Pd portion, as determined by X-ray photoelectron spectroscopy, electrochemical surface area, and half-wave potential measurements, respectively. Among the Pd/C, Pd4Ir/C and Pd–Ir ternary alloy catalysts, Pd4IrY0.1/C exhibited the largest electrochemical surface area (26.46 m2/gPd) and the highest half-wave potential (0.626 V). In particular, the metallic Pd portion in the ternary alloy had a stronger relationship with the activity towards the ORR than the electrochemical surface area. Therefore, in order to enhance the ORR activity of Pd-based alloy catalysts, the alloying method, which reduces more rare-earth elements, should be developed to increase the metallic Pd portion.
中文翻译:

钯三元合金中稀土元素对氧还原反应活性的影响
为了增强Pd对氧还原反应(ORR)的催化活性,将过渡金属与Pd合金化。这会改变Pd的电子结构,因为合金化的过渡金属会引起晶格失配和配体效应。稀土元素的电负性要低得多,因此可以轻易地给电子并填充Pd的反键轨道。因此,稀土元素通过使Pd更具金属性而有效地提高了ORR活性。此外,使Ir与贵金属合金化可通过在酸性介质中的应变效应来增强ORR活性。在这项研究中,使用多元醇工艺将稀土元素(例如Y,Sc和La)与Pd和Ir合金化。由于稀土元素的原子尺寸比Pd的原子大,还原电位低得多,通过多元醇方法可将含稀土元素的Pd基合金催化剂的掺入量限制。稀土元素的添加增加了金属钯的含量;然而,当过量添加时,表面上的大多数稀土元素以氧化物的形式位于晶界中。当Pd,Ir和稀土元素的组成比为4:1:0.1时,由X确定,Pd–Ir–Y,Pd–Ir–La和Pd–Ir–Sc合金催化剂具有较高的金属Pd部分射线光电子能谱,电化学表面积和半波电势测量。在Pd / C中,Pd 表面上的大多数稀土元素以氧化物的形式位于晶界中。当Pd,Ir和稀土元素的组成比为4:1:0.1时,由X确定,Pd–Ir–Y,Pd–Ir–La和Pd–Ir–Sc合金催化剂具有较高的金属Pd部分射线光电子能谱,电化学表面积和半波电势测量。在Pd / C中,Pd 表面上的大多数稀土元素以氧化物的形式位于晶界中。当Pd,Ir和稀土元素的组成比为4:1:0.1时,由X确定,Pd–Ir–Y,Pd–Ir–La和Pd–Ir–Sc合金催化剂具有较高的金属Pd部分射线光电子能谱,电化学表面积和半波电势测量。在Pd / C中,Pd4 Ir / C和Pd-Ir三元合金催化剂Pd 4 IrY 0.1 / C表现出最大的电化学表面积(26.46 m 2 / g Pd)和最高的半波电势(0.626 V)。特别地,与电化学表面积相比,三元合金中的金属Pd部分与对ORR的活性具有更强的关系。因此,为了增强Pd基合金催化剂的ORR活性,应当开发减少更多稀土元素的合金化方法以增加金属Pd含量。











































 京公网安备 11010802027423号
京公网安备 11010802027423号