当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic Study on Ring‐Contracting Skeletal Rearrangement from Porphycene to Isocorrole by Experimental and Theoretical Methods
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-23 , DOI: 10.1002/ejoc.201901659 Taro Koide 1 , Takafumi Maeda 1 , Tsukasa Abe 2 , Yoshihito Shiota 2 , Yoshio Yano 1 , Toshikazu Ono 1, 3 , Kazunari Yoshizawa 2, 3 , Yoshio Hisaeda 1, 3
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-23 , DOI: 10.1002/ejoc.201901659 Taro Koide 1 , Takafumi Maeda 1 , Tsukasa Abe 2 , Yoshihito Shiota 2 , Yoshio Yano 1 , Toshikazu Ono 1, 3 , Kazunari Yoshizawa 2, 3 , Yoshio Hisaeda 1, 3
Affiliation
|
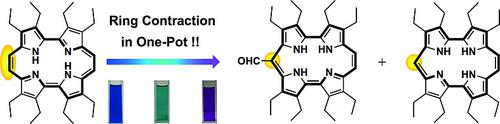
The ring‐contracting aromatic to aromatic skeletal rearrangement reaction from β‐octaethylporphycene to two‐types of isocorroles in one pot under basic conditions was confirmed. The electrochemical and photochemical properties were dramatically changed by the ring contraction. The mechanism of this unique reaction was proposed on the basis of the results of experimental and theoretical studies.

中文翻译:

实验和理论方法研究从卟啉到异戊二烯的缩环骨架重排的机理
证实了在碱性条件下,一锅中从β-八乙基卟啉到两种异氰酸酯的缩环芳烃到芳烃骨架重排反应。环收缩极大地改变了电化学和光化学性质。根据实验和理论研究的结果,提出了这种独特反应的机理。

更新日期:2020-01-23

中文翻译:

实验和理论方法研究从卟啉到异戊二烯的缩环骨架重排的机理
证实了在碱性条件下,一锅中从β-八乙基卟啉到两种异氰酸酯的缩环芳烃到芳烃骨架重排反应。环收缩极大地改变了电化学和光化学性质。根据实验和理论研究的结果,提出了这种独特反应的机理。




























 京公网安备 11010802027423号
京公网安备 11010802027423号