当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective N-Alkylation of Indoles in Aqueous Microdroplets.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-16 , DOI: 10.1002/anie.201913069 Elumalai Gnanamani 1, 2 , Xin Yan 1, 3 , Richard N Zare 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-01-16 , DOI: 10.1002/anie.201913069 Elumalai Gnanamani 1, 2 , Xin Yan 1, 3 , Richard N Zare 1, 2
Affiliation
|
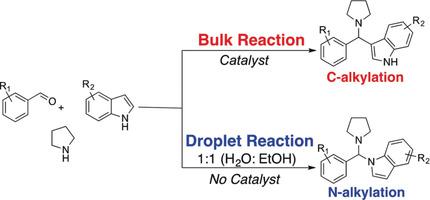
Many reactions show much faster kinetics in microdroplets than in the bulk phase. Most reported reactions in microdroplets mirror the products found in bulk reactions. However, the unique environment of microdroplets allows different chemistry to occur. In this work, we present the first chemoselective N-alkylation of indoles in aqueous microdroplets via a three-component Mannich-type reaction without using any catalyst. In sharp contrast, bulk reactions using the same reagents with a catalyst yield exclusively C-alkylation products. The N-alkylation yield is moderate in microdroplets, up to 53 %. We extended the scope of the microdroplet reaction and obtained a series of new functionalized indole aminals, which are likely to have biological activities. This work clearly indicates that microdroplet reactions can show reactivity quite different from that of bulk-phase reactions, which holds great potential for developing novel reactivities in microdroplets.
中文翻译:

在微滴水溶液中吲哚的化学选择性N-烷基化。
与微相相比,许多反应在微滴中的动力学要快得多。在微滴中报告的大多数反应反映了在本体反应中发现的产物。但是,微滴的独特环境允许发生不同的化学反应。在这项工作中,我们介绍了三组分曼尼希型反应在不使用任何催化剂的情况下,水性微滴中吲哚的第一个化学选择性N-烷基化反应。与之形成鲜明对比的是,使用相同试剂与催化剂的本体反应仅产生C-烷基化产物。在微滴中,N-烷基化产率中等,最高可达53%。我们扩展了微滴反应的范围,并获得了一系列可能具有生物学活性的新型功能化的吲哚类缩醛。
更新日期:2020-01-16
中文翻译:

在微滴水溶液中吲哚的化学选择性N-烷基化。
与微相相比,许多反应在微滴中的动力学要快得多。在微滴中报告的大多数反应反映了在本体反应中发现的产物。但是,微滴的独特环境允许发生不同的化学反应。在这项工作中,我们介绍了三组分曼尼希型反应在不使用任何催化剂的情况下,水性微滴中吲哚的第一个化学选择性N-烷基化反应。与之形成鲜明对比的是,使用相同试剂与催化剂的本体反应仅产生C-烷基化产物。在微滴中,N-烷基化产率中等,最高可达53%。我们扩展了微滴反应的范围,并获得了一系列可能具有生物学活性的新型功能化的吲哚类缩醛。











































 京公网安备 11010802027423号
京公网安备 11010802027423号