当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coligand driven diverse organometallation in benzothiazolyl-hydrazone derivatized pyrene: ortho vs. peri C–H activation†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-12-16 , DOI: 10.1039/c9nj05088d Soumitra Dinda 1, 2, 3, 4 , Sarat Chandra Patra 1, 4, 5, 6, 7 , Subhadip Roy 1, 4, 8 , Supriyo Halder 1, 4, 5, 6, 7 , Thomas Weyhermüller 9, 10, 11 , Kausikisankar Pramanik 1, 4, 5, 6, 7 , Sanjib Ganguly 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-12-16 , DOI: 10.1039/c9nj05088d Soumitra Dinda 1, 2, 3, 4 , Sarat Chandra Patra 1, 4, 5, 6, 7 , Subhadip Roy 1, 4, 8 , Supriyo Halder 1, 4, 5, 6, 7 , Thomas Weyhermüller 9, 10, 11 , Kausikisankar Pramanik 1, 4, 5, 6, 7 , Sanjib Ganguly 1, 2, 3, 4
Affiliation
|
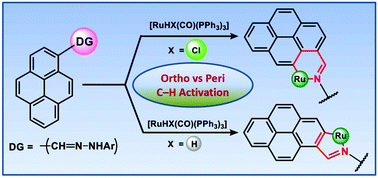
Benzothiazolyl hydrazones 1 (H2LPAH) incorporating polycyclic aromatic hydrocarbons (PAHs) have been fabricated as hemilabile scaffolds and elegantly utilized the inbuilt nitrogen donors as a proficient directing group (DG) to bring about ruthenium(II) assisted C–H activation in PAHs at both the peri and ortho positions. An isomeric pair of organometallics having formula [RuII(LPyr)(CO)(PPh3)2] (peri: 3a, ortho: 5a) have been conveniently prepared by varying the [RuII] precursors with H2LPyr. In contrast, only one type of activated product viz. [RuII(LAnc)(CO)(PPh3)2] 3b has been obtained with the 9-anthracene derivative of 1, H2LAnc, under analogous reaction conditions. The underlying mechanistic aspects have been elucidated by isolating the thermally unstable intermediates viz. [RuII(HLPyr)Cl(CO)(PPh3)2] 2a and [RuII(HLPyr)H(CO)(PPh3)2] 4a in the course of the peri and ortho C–H activation processes, respectively. The coligand (Cl/H) plays a vital role to bring about the C–H activation at the desired positions via formation of either a four- or five-membered metallacycle in 2a and 4a, respectively. The activation process vis-à-vis Ru–C bond formation in 3a can be achieved smoothly from 2a by a thermal transformation route, which proceeds via an initial rupture of the Ru–Nhydrazonyl bond. In contrast, the trans influential hydride coligand prefers a five-membered chelate to avoid confrontation with Nhydrazone in 4a, which in turn furnishes exclusively ortho activation owing to the close approach of the Ru–H bond towards ortho-H in pyrene. The organometallated complexes exhibit oxidative responses at mild potential. EPR and computational studies indicate that the redox activity originates from the ligand-centered orbitals. The observed rich optoelectronic features are analysed primarily as 1ILCT admixed with 1MLCT components by theoretical means, indicating an appreciable accumulation of electron density over the ligand backbone in their ground states.
中文翻译:

配位体驱动的苯并噻唑基hydr pyr衍生的diverse中的各种有机金属化:邻位vs. CHH活化†
结合了多环芳烃(PAHs)的苯并噻唑1(H 2 L PAH)被制成半不稳定的支架,并优雅地利用内置的氮供体作为熟练的导向基团(DG)来实现钌(II)辅助C–H活化周围和邻位的PAHs 。通过改变[Ru]可以方便地制备具有式[Ru II(L Pyr)(CO)(PPh 3)2 ](peri:3a,ortho:5a)的有机金属异构体对。二]前体与H 2 L的Pyr。相反,仅一种类型的活化产物即。[Ru II(L Anc)(CO)(PPh 3) 2 ] 3b在类似的反应条件下用1的9-蒽衍生物H 2 L Anc得到。底层机制方面已通过隔离热不稳定的中间体阐明即。[Ru II(HL Pyr)Cl(CO)(PPh 3) 2 ] 2a和[Ru II(HLPyr)H(CO)(PPh 3) 2 ] 4a分别在周围和邻位C–H活化过程中发生。大肠菌素(Cl / H)通过在2a和4a中分别形成四元或五元金属环,在所需位置实现CH活化起着至关重要的作用。相对于Ru–C键在3a中形成的活化过程可以通过热转化途径从2a顺利完成,该过程通过Ru–N肼基键的初始断裂而进行。相反,反式有影响氢化共配体偏好的五元螯合物到用N避免对抗腙在4a中,而这又配料专门邻由于朝向的Ru-H键的紧密接近激活邻芘-H。有机金属化的配合物在中等电位下表现出氧化反应。EPR和计算研究表明,氧化还原活性起源于以配体为中心的轨道。观察到的丰富的光电特征主要是通过理论方法将1 ILCT与1 MLCT组分混合在一起进行分析,表明在配体主链上处于基态的电子密度有明显的积累。
更新日期:2020-01-14
中文翻译:

配位体驱动的苯并噻唑基hydr pyr衍生的diverse中的各种有机金属化:邻位vs. CHH活化†
结合了多环芳烃(PAHs)的苯并噻唑1(H 2 L PAH)被制成半不稳定的支架,并优雅地利用内置的氮供体作为熟练的导向基团(DG)来实现钌(II)辅助C–H活化周围和邻位的PAHs 。通过改变[Ru]可以方便地制备具有式[Ru II(L Pyr)(CO)(PPh 3)2 ](peri:3a,ortho:5a)的有机金属异构体对。二]前体与H 2 L的Pyr。相反,仅一种类型的活化产物即。[Ru II(L Anc)(CO)(PPh 3) 2 ] 3b在类似的反应条件下用1的9-蒽衍生物H 2 L Anc得到。底层机制方面已通过隔离热不稳定的中间体阐明即。[Ru II(HL Pyr)Cl(CO)(PPh 3) 2 ] 2a和[Ru II(HLPyr)H(CO)(PPh 3) 2 ] 4a分别在周围和邻位C–H活化过程中发生。大肠菌素(Cl / H)通过在2a和4a中分别形成四元或五元金属环,在所需位置实现CH活化起着至关重要的作用。相对于Ru–C键在3a中形成的活化过程可以通过热转化途径从2a顺利完成,该过程通过Ru–N肼基键的初始断裂而进行。相反,反式有影响氢化共配体偏好的五元螯合物到用N避免对抗腙在4a中,而这又配料专门邻由于朝向的Ru-H键的紧密接近激活邻芘-H。有机金属化的配合物在中等电位下表现出氧化反应。EPR和计算研究表明,氧化还原活性起源于以配体为中心的轨道。观察到的丰富的光电特征主要是通过理论方法将1 ILCT与1 MLCT组分混合在一起进行分析,表明在配体主链上处于基态的电子密度有明显的积累。











































 京公网安备 11010802027423号
京公网安备 11010802027423号