当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal–organic architectures driven by a multifunctional 6-aminouracil spacer: structures, noncovalent interactions, and conductivity
CrystEngComm ( IF 2.6 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9ce01437c Atanu Purkayastha 1, 2, 3 , Sourab Dhar 2, 3, 4 , Suvra Prakash Mondal 2, 3, 4 , Antonio Franconetti 5, 6, 7 , Antonio Frontera 5, 6, 7 , Rakesh Ganguly 8, 9, 10 , Alexander M. Kirillov 11, 12, 13, 14, 15 , Tarun Kumar Misra 1, 2, 3
CrystEngComm ( IF 2.6 ) Pub Date : 2019/12/16 , DOI: 10.1039/c9ce01437c Atanu Purkayastha 1, 2, 3 , Sourab Dhar 2, 3, 4 , Suvra Prakash Mondal 2, 3, 4 , Antonio Franconetti 5, 6, 7 , Antonio Frontera 5, 6, 7 , Rakesh Ganguly 8, 9, 10 , Alexander M. Kirillov 11, 12, 13, 14, 15 , Tarun Kumar Misra 1, 2, 3
Affiliation
|
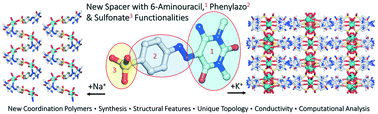
Three new coordination polymers, [NaI(H2L)(H2O)3]n (1), {[KI(H2L)(H2O)]8·13H2O}n (2), and {[NiIINaI2(HL)2(H2O)8]·6H2O}n (3), were assembled from 1,3-dimethyl-5-(p-sulfonic-phenylazo)-6-aminouracil (H3L) as a versatile building block. Although azo-coupling reactions generally result in simple azo-compounds, the presence of the sulfonate (–SO3−) group at the ligand frame led to the formation of the NaI-1D (1) or KI-3D (2) homometallic coordination polymer with different ligand coordination modes. Moreover, the heterometallic NiII/NaI-1D (3) coordination polymer was obtained while carrying the reaction of 2 with NiCl2·6H2O. The structures of the obtained products were fully established by single crystal X-ray diffraction and confirmed by standard methods. Compounds 1 and 3 possess 1D metal–organic chains with the 2C1 topology, whereas 2 features a very complex 3D metal–organic architecture with an unprecedented topology. The computational study for molecular electrostatic potential (MEP) surface energies revealed an important finding, namely a decrease of the π-acidity of the uracil ring upon coordination and a consequent increase of the π-basicity of the phenyl-sulfonate ring, resulting in effective anti-parallel π–π stacking interactions in 1 and 2. Finally, a semi-conductive behavior of the obtained compounds was explored using impedance spectroscopy, revealing the very remarkable conductive properties of 1 (2.2 × 10−4 S cm−1), which is a far better conductive material than 2 (7.2 × 10−6 S cm−1) and 3 (4.8 × 10−7 S cm−1). The obtained products represent the first coordination compounds derived from H3L. This study contributes to the design of functional coordination polymers driven by still poorly explored 6-aminouracil building blocks.
中文翻译:

多功能6-氨基尿嘧啶间隔子驱动的金属有机结构:结构,非共价相互作用和电导率
三种新的配位聚合物,[Na I(H 2 L)(H 2 O)3 ] n(1),{[K I(H 2 L)(H 2 O)] 8 ·13H 2 O} n(2) ,和{[Ni II Na I 2(HL)2(H 2 O)8 ]·6H 2 O} n(3)由1,3-二甲基-5-(对-磺酸-苯偶氮)-6组装而成-氨基尿嘧啶(H 3L)作为通用构建块。虽然偶氮偶联反应通常导致简单偶氮化合物,磺酸根的存在(-SO 3 - )基团在配位体框架导致的Na的形成我-1D(1)或K我-3D(2)配体配位方式不同的均金属配位聚合物 此外,在使2与NiCl 2 ·6H 2进行反应的同时,获得了杂金属Ni II / Na I -1D(3)配位聚合物。O.获得的产物的结构通过单晶X射线衍射完全确定并通过标准方法确认。化合物1和3具有2C1拓扑的一维金属有机链,而化合物2具有非常复杂的3D金属有机结构,具有前所未有的拓扑。分子静电势(MEP)表面能的计算研究显示出一个重要发现,即配位时尿嘧啶环的π酸度降低,以及苯磺酸盐环的π碱度增加,从而产生了有效1和2中的反平行π–π堆积相互作用。最后,使用阻抗谱研究了所得化合物的半导电行为,发现其非常出色的导电性能1(2.2×10 -4 S cm -1)比2(7.2×10)更好。-6 S cm -1)和3(4.8×10 -7 S cm -1)。所获得的产物代表了衍生自H 3 L的第一个配位化合物。这项研究有助于设计功能性配位聚合物,而该聚合物仍由探索不佳的6-氨基尿嘧啶结构单元驱动。
更新日期:2020-02-10
中文翻译:

多功能6-氨基尿嘧啶间隔子驱动的金属有机结构:结构,非共价相互作用和电导率
三种新的配位聚合物,[Na I(H 2 L)(H 2 O)3 ] n(1),{[K I(H 2 L)(H 2 O)] 8 ·13H 2 O} n(2) ,和{[Ni II Na I 2(HL)2(H 2 O)8 ]·6H 2 O} n(3)由1,3-二甲基-5-(对-磺酸-苯偶氮)-6组装而成-氨基尿嘧啶(H 3L)作为通用构建块。虽然偶氮偶联反应通常导致简单偶氮化合物,磺酸根的存在(-SO 3 - )基团在配位体框架导致的Na的形成我-1D(1)或K我-3D(2)配体配位方式不同的均金属配位聚合物 此外,在使2与NiCl 2 ·6H 2进行反应的同时,获得了杂金属Ni II / Na I -1D(3)配位聚合物。O.获得的产物的结构通过单晶X射线衍射完全确定并通过标准方法确认。化合物1和3具有2C1拓扑的一维金属有机链,而化合物2具有非常复杂的3D金属有机结构,具有前所未有的拓扑。分子静电势(MEP)表面能的计算研究显示出一个重要发现,即配位时尿嘧啶环的π酸度降低,以及苯磺酸盐环的π碱度增加,从而产生了有效1和2中的反平行π–π堆积相互作用。最后,使用阻抗谱研究了所得化合物的半导电行为,发现其非常出色的导电性能1(2.2×10 -4 S cm -1)比2(7.2×10)更好。-6 S cm -1)和3(4.8×10 -7 S cm -1)。所获得的产物代表了衍生自H 3 L的第一个配位化合物。这项研究有助于设计功能性配位聚合物,而该聚合物仍由探索不佳的6-氨基尿嘧啶结构单元驱动。









































 京公网安备 11010802027423号
京公网安备 11010802027423号