当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Opposite responses of normal hepatocytes and hepatocellular carcinoma cells to substrate viscoelasticity.
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9bm01339c Kalpana Mandal 1 , Ze Gong , Alexis Rylander , Vivek B Shenoy , Paul A Janmey
Biomaterials Science ( IF 5.8 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9bm01339c Kalpana Mandal 1 , Ze Gong , Alexis Rylander , Vivek B Shenoy , Paul A Janmey
Affiliation
|
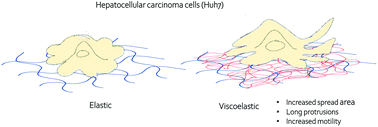
The cellular microenvironment plays a critical role in cell differentiation, proliferation, migration, and homeostasis. Recent studies have shown the importance of substrate viscosity in determining cellular function. Here, we study the mechanoresponse of normal hepatocytes and hepatocellular carcinoma cells (HCC) to elastic and viscoelastic substrates using the Huh7 cell line derived from a human liver tumor and primary human hepatocytes (PHH). Unlike PHH and fibroblasts, which respond to viscoelastic substrates by reducing spreading area and actin bundle assembly compared to purely elastic substrates of the same stiffness, Huh7 cells spread faster on viscoelastic substrates than on purely elastic substrates. The steady state spreading areas of Huh7 cells are larger on viscoelastic substrates, whereas the opposite effect occurs with PHH cells. The viscoelasticity of the microenvironment also promotes motility and multiple long protrusions in Huh7 cells. Pharmacologic disruption of the actin assembly makes cells unable to spread on either elastic or viscoelastic substrates. In contrast, upon vimentin perturbation, cells still spread to a limited degree on elastic substrates but are unable to spread on viscoelastic substrates. The time evolution of cell traction force shows that the peak occurs at an earlier time point on viscoelastic substrates compared to elastic substrates. However, the total force generation at steady state is the same on both substrates after 4 hours. Our data suggest that stress relaxation time scales of the viscoelastic substrate regulate cell dynamics and traction force generation, indicating different binding-unbinding rates of the proteins that form cell attachment sites in HCC cells and normal hepatocytes. These results suggest that liver cancer cells may have different characteristic lifetimes of binding to the substrate in comparision to normal cells, which might cause differences in cell spreading and motility within the diseased tissue.
中文翻译:

正常肝细胞和肝细胞癌细胞对基质粘弹性的相反反应。
细胞微环境在细胞分化,增殖,迁移和体内稳态中起关键作用。最近的研究表明底物粘度在确定细胞功能中的重要性。在这里,我们使用人类肝肿瘤和原代人肝细胞(PHH)衍生的Huh7细胞系研究正常肝细胞和肝细胞癌细胞(HCC)对弹性和粘弹性底物的机械反应。与具有相同刚度的纯弹性基底相比,PHH和成纤维细胞通过减少扩散面积和肌动蛋白束组装来响应粘弹性基底,而Huh7细胞在粘弹性基底上的扩散比在纯弹性基底上的扩散快。在粘弹性基质上,Huh7细胞的稳态扩散区域更大,而PHH细胞则发生相反的作用。微环境的粘弹性还促进Huh7细胞的运动性和多个长突起。肌动蛋白装配体的药理学破坏使细胞无法在弹性或粘弹性底物上扩散。相反,在波形蛋白微扰后,细胞仍在弹性基底上以有限的程度扩散,但不能在粘弹性基底上扩散。细胞牵引力的时间演变表明,与弹性基材相比,粘弹性基材上的峰值出现在更早的时间点。但是,在4小时后,两个基板在稳态下产生的总力相同。我们的数据表明,粘弹性基材的应力松弛时间尺度可调节细胞动力学和牵引力的产生,这表明在HCC细胞和正常肝细胞中形成细胞附着位点的蛋白质的结合解除结合率不同。这些结果表明,与正常细胞相比,肝癌细胞可能具有与底物结合的不同特征寿命,这可能引起患病组织内细胞扩散和运动性的差异。
更新日期:2020-03-03
中文翻译:

正常肝细胞和肝细胞癌细胞对基质粘弹性的相反反应。
细胞微环境在细胞分化,增殖,迁移和体内稳态中起关键作用。最近的研究表明底物粘度在确定细胞功能中的重要性。在这里,我们使用人类肝肿瘤和原代人肝细胞(PHH)衍生的Huh7细胞系研究正常肝细胞和肝细胞癌细胞(HCC)对弹性和粘弹性底物的机械反应。与具有相同刚度的纯弹性基底相比,PHH和成纤维细胞通过减少扩散面积和肌动蛋白束组装来响应粘弹性基底,而Huh7细胞在粘弹性基底上的扩散比在纯弹性基底上的扩散快。在粘弹性基质上,Huh7细胞的稳态扩散区域更大,而PHH细胞则发生相反的作用。微环境的粘弹性还促进Huh7细胞的运动性和多个长突起。肌动蛋白装配体的药理学破坏使细胞无法在弹性或粘弹性底物上扩散。相反,在波形蛋白微扰后,细胞仍在弹性基底上以有限的程度扩散,但不能在粘弹性基底上扩散。细胞牵引力的时间演变表明,与弹性基材相比,粘弹性基材上的峰值出现在更早的时间点。但是,在4小时后,两个基板在稳态下产生的总力相同。我们的数据表明,粘弹性基材的应力松弛时间尺度可调节细胞动力学和牵引力的产生,这表明在HCC细胞和正常肝细胞中形成细胞附着位点的蛋白质的结合解除结合率不同。这些结果表明,与正常细胞相比,肝癌细胞可能具有与底物结合的不同特征寿命,这可能引起患病组织内细胞扩散和运动性的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号