当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nilotinib Effects on Safety, Tolerability, and Potential Biomarkers in Parkinson Disease: A Phase 2 Randomized Clinical Trial.
JAMA Neurology ( IF 20.4 ) Pub Date : 2019-12-16 , DOI: 10.1001/jamaneurol.2019.4200 Fernando L Pagan 1, 2 , Michaeline L Hebron 1 , Barbara Wilmarth 1, 2 , Yasar Torres-Yaghi 1, 2 , Abigail Lawler 1, 2 , Elizabeth E Mundel 1, 2 , Nadia Yusuf 1, 2 , Nathan J Starr 1, 2 , Muhammad Anjum 1, 2 , Joy Arellano 2 , Helen H Howard 2 , Wangke Shi 1 , Sanjana Mulki 1 , Tarick Kurd-Misto 1 , Sara Matar 1 , Xiaoguang Liu 1 , Jaeil Ahn 3 , Charbel Moussa 1, 2
JAMA Neurology ( IF 20.4 ) Pub Date : 2019-12-16 , DOI: 10.1001/jamaneurol.2019.4200 Fernando L Pagan 1, 2 , Michaeline L Hebron 1 , Barbara Wilmarth 1, 2 , Yasar Torres-Yaghi 1, 2 , Abigail Lawler 1, 2 , Elizabeth E Mundel 1, 2 , Nadia Yusuf 1, 2 , Nathan J Starr 1, 2 , Muhammad Anjum 1, 2 , Joy Arellano 2 , Helen H Howard 2 , Wangke Shi 1 , Sanjana Mulki 1 , Tarick Kurd-Misto 1 , Sara Matar 1 , Xiaoguang Liu 1 , Jaeil Ahn 3 , Charbel Moussa 1, 2
Affiliation
|
Importance
This study evaluated nilotinib safety and its effects on biomarkers as a potential disease-modifying drug in Parkinson disease.
Objectives
To assess nilotinib effects on safety and pharmacokinetics and measure the change in exploratory biomarkers in patients with moderately severe Parkinson disease.
Design, Setting, and Participants
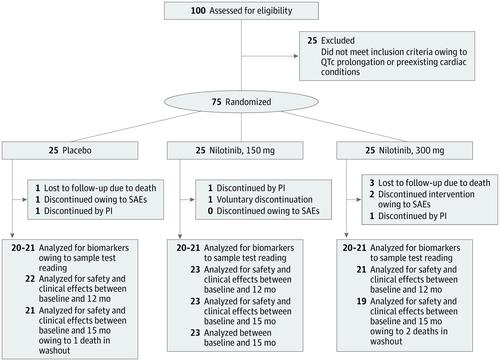
This was a single-center, phase 2, randomized, double-blind, placebo-controlled trial with 300 patients approached in clinic; of these, 200 declined to participate, 100 were screened, 25 were excluded, and 75 were randomized 1:1:1 into placebo; nilotinib, 150-mg; or nilotinib, 300-mg groups. Recruitment started on May 17, 2017, and ended April 28, 2018, and follow-up ended August 10, 2019. Parkinson disease was confirmed according to the UK Brain Bank diagnostic criteria and symptoms were stabilized with use of optimal levodopa and/or dopamine agonists and other medications used in Parkinson disease.
Interventions
Nilotinib vs placebo, administered orally once daily for 12 months followed by a 3-month washout period.
Main Outcomes and Measures
It was hypothesized that nilotinib is safe and can be detected in the cerebrospinal fluid, where it alters exploratory biomarkers via inhibition of Abelson tyrosine kinase and potentially improves clinical outcomes.
Results
Of the 75 patients included in the study, 55 were men (73.3%); mean (SD) age was 68.4 (8.2) years. Doses of 150 or 300 mg of nilotinib were reasonably safe, although more serious adverse events were detected in the nilotinib (150 mg: 6 [24%]; 300 mg: 12 [48%]) vs placebo (4 [16%]) groups. The 150-mg nilotinib group showed an increase in cerebrospinal fluid levels of the dopamine metabolites homovanillic acid (159.80nM; 90% CI, 7.04-312.60nM; P = .04) and 3,4-dihydroxyphenylacetic acid (4.87nM; 90% CI, 1.51-8.23nM; P = .01), and the 300-mg nilotinib group showed an increase in 3,4-dihydroxyphenylacetic acid (7.52nM; 90% CI, 2.35-12.69nM; P = .01). The nilotinib 150-mg but not the nilotinib 300-mg group demonstrated a reduction of α-synuclein oligomers (-0.04 pg/mL; 90% CI, -0.08 to 0.01 pg/mL; P = .03). A significant reduction of hyperphosphorylated tau levels was seen in the nilotinib 150-mg (-10.04 pg/mL; 90% CI, -17.41 to -2.67 pg/mL; P = .01) and nilotinib 300-mg (-12.05 pg/mL; 90% CI, -19.21 to -4.90 pg/mL; P = .01) groups.
Conclusions and Relevance
In this study, nilotinib appeared to be reasonably safe and detectable in the cerebrospinal fluid. Exploratory biomarkers were altered in response to nilotinib. Taken together, these data will guide the development of a phase 3 study to investigate the effects of nilotinib therapy in patients with Parkinson disease.
Trial Registration
ClinicalTrials.gov identifier: NCT02954978.
中文翻译:

尼罗替尼对帕金森病安全性、耐受性和潜在生物标志物的影响:2 期随机临床试验。
重要性 本研究评估了尼罗替尼作为帕金森病潜在疾病缓解药物的安全性及其对生物标志物的影响。目的 评估尼罗替尼对安全性和药代动力学的影响,并测量中重度帕金森病患者探索性生物标志物的变化。设计、设置和参与者 其中,200 人拒绝参与,100 人被筛选,25 人被排除,75 人以 1:1:1 随机分配到安慰剂组;尼罗替尼,150-mg;或尼罗替尼,300 毫克组。招募于2017年5月17日开始,至2018年4月28日结束,随访至2019年8月10日结束。根据英国脑库诊断标准确认帕金森病,并通过使用最佳左旋多巴和/或多巴胺激动剂和其他用于帕金森病的药物稳定症状。干预措施 尼罗替尼 vs 安慰剂,每天口服一次,持续 12 个月,然后是 3 个月的清除期。主要结果和措施 假设尼罗替尼是安全的,可以在脑脊液中检测到,它通过抑制 Abelson 酪氨酸激酶改变探索性生物标志物,并可能改善临床结果。结果 纳入研究的 75 名患者中,男性 55 人(73.3%);平均 (SD) 年龄为 68.4 (8.2) 岁。150 或 300 mg 的尼罗替尼剂量相当安全,尽管在尼罗替尼中检测到更严重的不良事件(150 mg:6 [24%];300 mg:12 [48%]) 与安慰剂 (4 [16%]) 组。150 mg 尼罗替尼组显示多巴胺代谢物高香草酸(159.80nM;90% CI,7.04-312.60nM;P = .04)和 3,4-二羟基苯乙酸(4.87nM;90%)的脑脊液水平升高CI,1.51-8.23nM;P = .01),300 mg 尼罗替尼组显示 3,4-二羟基苯乙酸增加(7.52nM;90% CI,2.35-12.69nM;P = .01)。尼罗替尼 150-mg 组而非尼罗替尼 300-mg 组显示 α-突触核蛋白寡聚体减少(-0.04 pg/mL;90% CI,-0.08 至 0.01 pg/mL;P = .03)。尼罗替尼 150-mg (-10.04 pg/mL; 90% CI, -17.41 至 -2.67 pg/mL; P = .01) 和尼罗替尼 300-mg (-12.05 pg/mL) 显着降低了过度磷酸化的 tau 水平mL;90% CI,-19.21 至 -4.90 pg/mL;P = .01) 组。结论和相关性 在这项研究中,尼罗替尼在脑脊液中似乎是相当安全和可检测的。探索性生物标志物响应尼罗替尼而改变。总之,这些数据将指导开展一项 3 期研究,以调查尼罗替尼治疗对帕金森病患者的影响。试验注册 ClinicalTrials.gov 标识符:NCT02954978。
更新日期:2020-03-09
中文翻译:

尼罗替尼对帕金森病安全性、耐受性和潜在生物标志物的影响:2 期随机临床试验。
重要性 本研究评估了尼罗替尼作为帕金森病潜在疾病缓解药物的安全性及其对生物标志物的影响。目的 评估尼罗替尼对安全性和药代动力学的影响,并测量中重度帕金森病患者探索性生物标志物的变化。设计、设置和参与者 其中,200 人拒绝参与,100 人被筛选,25 人被排除,75 人以 1:1:1 随机分配到安慰剂组;尼罗替尼,150-mg;或尼罗替尼,300 毫克组。招募于2017年5月17日开始,至2018年4月28日结束,随访至2019年8月10日结束。根据英国脑库诊断标准确认帕金森病,并通过使用最佳左旋多巴和/或多巴胺激动剂和其他用于帕金森病的药物稳定症状。干预措施 尼罗替尼 vs 安慰剂,每天口服一次,持续 12 个月,然后是 3 个月的清除期。主要结果和措施 假设尼罗替尼是安全的,可以在脑脊液中检测到,它通过抑制 Abelson 酪氨酸激酶改变探索性生物标志物,并可能改善临床结果。结果 纳入研究的 75 名患者中,男性 55 人(73.3%);平均 (SD) 年龄为 68.4 (8.2) 岁。150 或 300 mg 的尼罗替尼剂量相当安全,尽管在尼罗替尼中检测到更严重的不良事件(150 mg:6 [24%];300 mg:12 [48%]) 与安慰剂 (4 [16%]) 组。150 mg 尼罗替尼组显示多巴胺代谢物高香草酸(159.80nM;90% CI,7.04-312.60nM;P = .04)和 3,4-二羟基苯乙酸(4.87nM;90%)的脑脊液水平升高CI,1.51-8.23nM;P = .01),300 mg 尼罗替尼组显示 3,4-二羟基苯乙酸增加(7.52nM;90% CI,2.35-12.69nM;P = .01)。尼罗替尼 150-mg 组而非尼罗替尼 300-mg 组显示 α-突触核蛋白寡聚体减少(-0.04 pg/mL;90% CI,-0.08 至 0.01 pg/mL;P = .03)。尼罗替尼 150-mg (-10.04 pg/mL; 90% CI, -17.41 至 -2.67 pg/mL; P = .01) 和尼罗替尼 300-mg (-12.05 pg/mL) 显着降低了过度磷酸化的 tau 水平mL;90% CI,-19.21 至 -4.90 pg/mL;P = .01) 组。结论和相关性 在这项研究中,尼罗替尼在脑脊液中似乎是相当安全和可检测的。探索性生物标志物响应尼罗替尼而改变。总之,这些数据将指导开展一项 3 期研究,以调查尼罗替尼治疗对帕金森病患者的影响。试验注册 ClinicalTrials.gov 标识符:NCT02954978。











































 京公网安备 11010802027423号
京公网安备 11010802027423号