当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atomistic Insights into Photoprotein Formation: Computational Prediction of the Properties of Coelenterazine and Oxygen Binding in Obelin
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2019-12-16 , DOI: 10.1002/jcc.26125 Thomas M Griffiths 1, 2, 3 , Aaron J Oakley 1, 2, 3 , Haibo Yu 1, 2, 3
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2019-12-16 , DOI: 10.1002/jcc.26125 Thomas M Griffiths 1, 2, 3 , Aaron J Oakley 1, 2, 3 , Haibo Yu 1, 2, 3
Affiliation
|
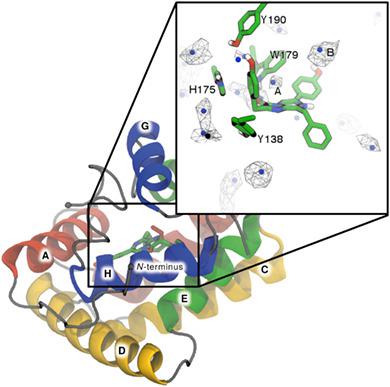
Bioluminescence in marine systems is dominated by the use of coelenterazine for light production. The bioluminescent reaction of coelenterazine is an enzyme catalyzed oxidative decarboxylation: coelenterazine reacts with molecular oxygen to form carbon dioxide, coelenteramide, and light. One such class is the Ca2+‐regulated photoproteins. These proteins bind coelenterazine and oxygen, and trap 2‐hydroperoxycoelenterazine, an intermediate along the reaction pathway. The reaction is halted until Ca2+ binding triggers the completion of the reaction. There are currently no reported experimental, atomistic descriptions of this ternary Michaelis complex. This study utilized computational techniques to develop an atomistic model of the Michaelis complex. Extensive molecular dynamics simulations were carried out to study the interactions between four tautomeric/protonation states of coelenterazine and wide‐type and mutant obelin. Only minor differences in binding modes were observed across all systems. Interestingly, no basic residues were identified in the vicinity of the N7‐nitrogen of coelenterazine. This observation was surprising considering that deprotonation at this position is a key mechanistic step in the proposed bioluminescent reaction. This work suggests that coelenterazine binds either as the O10H tautomer, or in the deprotonated form. Implicit ligand sampling simulations were used to identify potential O2 binding and migration pathways within obelin. A key oxygen binding site was identified close to the coelenterazine imidazopyrazinone core. The O2 binding free energy was observed to be dependent on the protonation state of coelenterazine. Taken together, the description of the obelin–coelenterazine‐O2 complexes established in this study provides the basis for future computational studies of the bioluminescent mechanism. © 2019 Wiley Periodicals, Inc.
中文翻译:

对光蛋白形成的原子洞察:对 Obelin 中腔肠素和氧结合特性的计算预测
海洋系统中的生物发光主要是使用腔肠素来产生光。腔肠素的生物发光反应是一种酶催化氧化脱羧作用:腔肠素与分子氧反应生成二氧化碳、腔肠酰胺和光。其中一类是 Ca2+ 调节的光蛋白。这些蛋白质结合腔肠素和氧,并捕获 2-氢过氧腔肠素,这是反应途径的中间体。反应停止,直到 Ca2+ 结合触发反应完成。目前还没有关于这种三元米氏复合物的实验性、原子性描述的报道。这项研究利用计算技术开发了米氏复合体的原子模型。进行了广泛的分子动力学模拟以研究腔肠素的四种互变异构/质子化状态与宽型和突变体 obelin 之间的相互作用。在所有系统中仅观察到结合模式的微小差异。有趣的是,在腔肠素的 N7-氮附近没有发现碱性残基。考虑到该位置的去质子化是所提出的生物发光反应中的关键机械步骤,这一观察结果令人惊讶。这项工作表明腔肠素以 O10H 互变异构体或去质子化形式结合。隐式配体采样模拟用于识别 obelin 内潜在的 O2 结合和迁移途径。在腔肠素咪唑并吡嗪酮核心附近鉴定了一个关键的氧结合位点。观察到 O2 结合自由能取决于腔肠素的质子化状态。总之,本研究中对 obelin-coelenterazine-O2 复合物的描述为未来生物发光机制的计算研究提供了基础。© 2019 威利期刊公司。
更新日期:2019-12-16
中文翻译:

对光蛋白形成的原子洞察:对 Obelin 中腔肠素和氧结合特性的计算预测
海洋系统中的生物发光主要是使用腔肠素来产生光。腔肠素的生物发光反应是一种酶催化氧化脱羧作用:腔肠素与分子氧反应生成二氧化碳、腔肠酰胺和光。其中一类是 Ca2+ 调节的光蛋白。这些蛋白质结合腔肠素和氧,并捕获 2-氢过氧腔肠素,这是反应途径的中间体。反应停止,直到 Ca2+ 结合触发反应完成。目前还没有关于这种三元米氏复合物的实验性、原子性描述的报道。这项研究利用计算技术开发了米氏复合体的原子模型。进行了广泛的分子动力学模拟以研究腔肠素的四种互变异构/质子化状态与宽型和突变体 obelin 之间的相互作用。在所有系统中仅观察到结合模式的微小差异。有趣的是,在腔肠素的 N7-氮附近没有发现碱性残基。考虑到该位置的去质子化是所提出的生物发光反应中的关键机械步骤,这一观察结果令人惊讶。这项工作表明腔肠素以 O10H 互变异构体或去质子化形式结合。隐式配体采样模拟用于识别 obelin 内潜在的 O2 结合和迁移途径。在腔肠素咪唑并吡嗪酮核心附近鉴定了一个关键的氧结合位点。观察到 O2 结合自由能取决于腔肠素的质子化状态。总之,本研究中对 obelin-coelenterazine-O2 复合物的描述为未来生物发光机制的计算研究提供了基础。© 2019 威利期刊公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号