Nature Chemical Biology ( IF 12.9 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41589-019-0423-2 Junhong Choi 1, 2, 3 , James Marks 4, 5 , Jingji Zhang 1 , Dong-Hua Chen 1 , Jinfan Wang 1 , Nora Vázquez-Laslop 4 , Alexander S Mankin 4 , Joseph D Puglisi 1
|
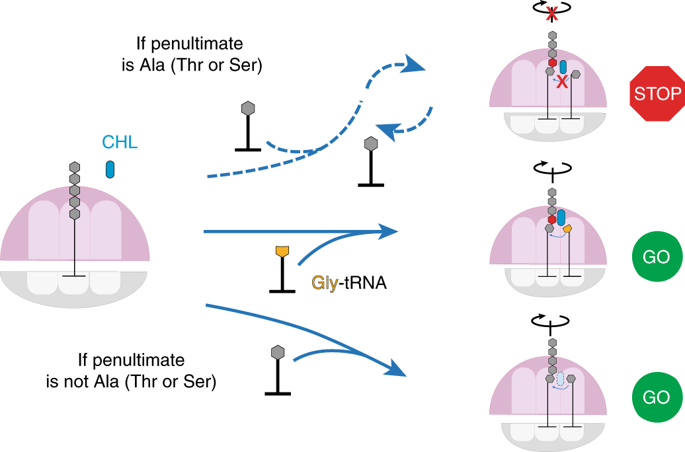
Chloramphenicol (CHL) and linezolid (LZD) are antibiotics that inhibit translation. Both were thought to block peptide-bond formation between all combinations of amino acids. Yet recently, a strong nascent peptide context-dependency of CHL- and LZD-induced translation arrest was discovered. Here we probed the mechanism of action of CHL and LZD by using single-molecule Förster resonance energy transfer spectroscopy to monitor translation arrest induced by antibiotics. The presence of CHL or LZD does not substantially alter dynamics of protein synthesis until the arrest-motif of the nascent peptide is generated. Inhibition of peptide-bond formation compels the fully accommodated A-site transfer RNA to undergo repeated rounds of dissociation and nonproductive rebinding. The glycyl amino-acid moiety on the A-site Gly-tRNA manages to overcome the arrest by CHL. Our results illuminate the mechanism of CHL and LZD action through their interactions with the ribosome, the nascent peptide and the incoming amino acid, perturbing elongation dynamics.
中文翻译:

氯霉素和利奈唑胺对上下文特异性翻译的抑制作用。
氯霉素(CHL)和利奈唑胺(LZD)是抑制翻译的抗生素。两者均被认为可阻止氨基酸的所有组合之间的肽键形成。然而,最近,发现了CHL和LZD诱导的翻译停滞强烈的新生肽上下文依赖性。在这里,我们通过使用单分子Förster共振能量转移光谱法监测抗生素诱导的翻译停滞,探讨了CHL和LZD的作用机理。CHL或LZD的存在基本上不会改变蛋白质合成的动力学,直到生成新生肽的阻滞基序。肽键形成的抑制迫使完全容纳的A位转移RNA经历重复的解离和非生产性重新结合。A位Gly-tRNA上的糖基氨基酸部分设法克服了CHL的阻滞作用。我们的研究结果阐明了CHL和LZD通过与核糖体,新生肽和传入氨基酸的相互作用作用的机理,扰乱了延伸动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号