当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconsidering the Structure of Serlyticin-A.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.jnatprod.9b00859 Ka Yi Tsui 1 , Robert J Tombari 1 , David E Olson 1, 2, 3 , Dean J Tantillo 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.jnatprod.9b00859 Ka Yi Tsui 1 , Robert J Tombari 1 , David E Olson 1, 2, 3 , Dean J Tantillo 1
Affiliation
|
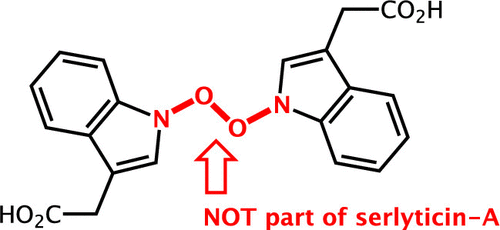
Serlyticin-A is a secondary metabolite first isolated from a culture of Serratia ureilytica grown using squid pen as the sole carbon/nitrogen source. A previous study by Kuo et al. demonstrated that it has antioxidative and antiproliferative properties. However, the proposed chemical structure of serlyticin-A is likely incorrect based on the thermodynamic instability of its three contiguous heteroatom-heteroatom bonds. Here, we use quantum chemical calculations to predict 1H and 13C chemical shifts for serlyticin-A and demonstrate a discrepancy between the calculated and experimental chemical shifts. We then propose several reasonable alternative structures for serlyticin-A. Considering the known antioxidant and antiproliferative activity of hydroxamic acids as well as their stability and prevalence in natural products of bacterial origin, we believe that serlyticin-A is most likely 3-indolylacetohydroxamic acid (4). We provide our rationale for this assignment as well as experimental data for pure 3-indolylacetohydroxamic acid obtained via de novo synthesis. This study highlights the power of computational NMR shift prediction to revise chemical structures for natural products like serlyticin-A.
中文翻译:

重新考虑 Serlyticin-A 的结构。
Serlyticin-A 是一种次生代谢产物,首先从使用鱿鱼圈作为唯一碳/氮源生长的解脲沙雷氏菌培养物中分离出来。 Kuo 等人之前的一项研究。证明它具有抗氧化和抗增殖特性。然而,基于其三个连续的杂原子-杂原子键的热力学不稳定性,所提出的丝利霉素-A的化学结构可能是不正确的。在这里,我们使用量子化学计算来预测 Serlyticin-A 的 1H 和 13C 化学位移,并证明计算的化学位移与实验的化学位移之间的差异。然后我们提出了 Serlyticin-A 的几种合理的替代结构。考虑到异羟肟酸已知的抗氧化和抗增殖活性及其在细菌来源的天然产物中的稳定性和普遍性,我们认为 Serlyticin-A 最有可能是 3-吲哚基乙酰异羟肟酸 (4)。我们提供了这项任务的基本原理以及通过从头合成获得的纯 3-吲哚基乙酰异羟肟酸的实验数据。这项研究强调了计算 NMR 位移预测在修改 Serlyticin-A 等天然产物的化学结构方面的能力。
更新日期:2019-12-17
中文翻译:

重新考虑 Serlyticin-A 的结构。
Serlyticin-A 是一种次生代谢产物,首先从使用鱿鱼圈作为唯一碳/氮源生长的解脲沙雷氏菌培养物中分离出来。 Kuo 等人之前的一项研究。证明它具有抗氧化和抗增殖特性。然而,基于其三个连续的杂原子-杂原子键的热力学不稳定性,所提出的丝利霉素-A的化学结构可能是不正确的。在这里,我们使用量子化学计算来预测 Serlyticin-A 的 1H 和 13C 化学位移,并证明计算的化学位移与实验的化学位移之间的差异。然后我们提出了 Serlyticin-A 的几种合理的替代结构。考虑到异羟肟酸已知的抗氧化和抗增殖活性及其在细菌来源的天然产物中的稳定性和普遍性,我们认为 Serlyticin-A 最有可能是 3-吲哚基乙酰异羟肟酸 (4)。我们提供了这项任务的基本原理以及通过从头合成获得的纯 3-吲哚基乙酰异羟肟酸的实验数据。这项研究强调了计算 NMR 位移预测在修改 Serlyticin-A 等天然产物的化学结构方面的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号