当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.actbio.2019.12.015 Chenglong Wang 1 , Wencai Guan 2 , Jinliang Peng 3 , Yuetan Chen 3 , Guoxiong Xu 2 , Hongjing Dou 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.actbio.2019.12.015 Chenglong Wang 1 , Wencai Guan 2 , Jinliang Peng 3 , Yuetan Chen 3 , Guoxiong Xu 2 , Hongjing Dou 1
Affiliation
|
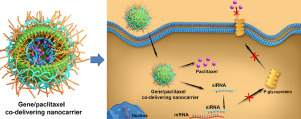
While drug resistance has been recognized as the main cause of unsuccessful chemotherapy, the efficient inhibition of highly drug-resistant tumors still remains a significant challenge, especially for in vivo treatments. Drug resistance has been associated with the high expression of the multi-drug resistance gene 1 (MDR1), which can encode an efflux transporter known as P-glycoprotein (P-gp) that is located in the cellular membrane. Therefore, the combined delivery of MDR1-inhibited genes and chemotherapeutic drugs is anticipated to enable the effective inhibition of drug-resistant tumors. Herein, highly paclitaxel (PTX)-resistant ovarian (OV) cancer with a drug resistance index reaching up to ~ 60 was chosen to evaluate the performance of an efficient gene/drug co-delivery nanocarrier. Inspired by the self-assembly that occurs in cells and exosomes, we designed a biomimetic lipid/dextran hybrid nanocarrier with a diameter of ~ 100 nm to enhance the endocytosis and the efficiency of drug/gene release within the cells. This nanocarrier was fabricated via the frame-guided self-assembly of lipid amphiphiles on the surfaces of redox-cleavable dextran-based nanogels. The anionic MDR1-siRNA and the hydrophobic drug PTX were respectively loaded into the cationic lipid shell and the hydrophobic internal core of the hybrid nanocarriers. MDR1-siRNA can knock down MDR1, promoting the accumulation of PTX in cells, and thus is expected to achieve an efficient inhibitory effect against highly PTX-resistant cancer cells. Both in vitro and in vivo studies revealed that this dual-delivery system significantly enhanced the therapeutic effect in comparison with that provided by a PTX-only system. Thus, the construction of gene/chemo co-delivered lipid/dextran nanocarriers provides a new strategy to inhibit highly drug-resistant tumors both in vitro and in vivo. In addition, this work will contribute toward the development of urgently needed tumor nanotherapy that is able to overcome drug resistance while also offering an unmatched range of effective therapeutic nanocarriers. STATEMENT OF SIGNIFICANCE: The biomimetic lipid/dextran hybrid nanocarrier with a diameter of ~ 100 nm, which was fabricated via the frame-guided self-assembly of lipid amphiphiles onto the surface of redox-cleavable dextran-based nanogels, provides a model carrier to co-deliver MDR1-siRNA and PTX. The MDR1-siRNA/PTX co-loaded biomimetic lipid/dextran hybrid nanocarriers demonstrate good capability in overcoming the PTX-resistance in highly chemo-resistant human ovarian (OV) cancer cells both in vitro and in vivo.
中文翻译:

通过框架诱导的自组装制备的基因/紫杉醇共递送纳米载体,用于抑制高度耐药的肿瘤。
尽管人们已经认识到耐药性是化疗失败的主要原因,但是有效抑制高度耐药性的肿瘤仍然是一项重大挑战,尤其是对于体内治疗而言。耐药与多药耐药基因1(MDR1)的高表达有关,该基因可以编码位于细胞膜中的称为P-糖蛋白(P-gp)的外排转运蛋白。因此,MDR1抑制基因和化学治疗药物的联合交付有望实现对耐药性肿瘤的有效抑制。在本文中,选择具有高达〜60的耐药指数的高度紫杉醇(PTX)耐药性卵巢癌(OV),以评估有效的基因/药物共递送纳米载体的性能。受细胞和外泌体中发生的自组装的启发,我们设计了直径约为100 nm的仿生脂质/葡聚糖杂化纳米载体,以增强细胞内吞作用和药物/基因释放的效率。该纳米载体是通过脂质两亲物在氧化还原可切割的葡聚糖基纳米凝胶表面上的框架引导的自组装而制备的。将阴离子MDR1-siRNA和疏水性药物PTX分别加载到杂化纳米载体的阳离子脂质壳和疏水性内核中。MDR1-siRNA可以敲低MDR1,促进细胞内PTX的积累,因此有望实现对高度PTX耐药的癌细胞的有效抑制作用。体外和体内研究均表明,与仅使用PTX的系统相比,这种双重递送系统显着增强了治疗效果。因此,基因/化学共递送脂质/葡聚糖纳米载体的构建提供了在体外和体内抑制高度耐药性肿瘤的新策略。此外,这项工作将有助于开发迫切需要的肿瘤纳米疗法,该疗法能够克服耐药性,同时还提供无与伦比的有效治疗纳米载体。意义声明:仿生脂质/葡聚糖杂化纳米载体,直径约100 nm,是通过脂质两亲物的框架引导自组装在氧化还原可切割的葡聚糖基纳米凝胶表面上制备的,提供了共同提供MDR1-siRNA和PTX的模型载体。MDR1-siRNA / PTX共同负载的仿生脂质/葡聚糖杂合纳米载体在体外和体内均显示出在高化学抗性人卵巢癌(OV)癌细胞中克服PTX抗性的良好能力。
更新日期:2019-12-17
中文翻译:

通过框架诱导的自组装制备的基因/紫杉醇共递送纳米载体,用于抑制高度耐药的肿瘤。
尽管人们已经认识到耐药性是化疗失败的主要原因,但是有效抑制高度耐药性的肿瘤仍然是一项重大挑战,尤其是对于体内治疗而言。耐药与多药耐药基因1(MDR1)的高表达有关,该基因可以编码位于细胞膜中的称为P-糖蛋白(P-gp)的外排转运蛋白。因此,MDR1抑制基因和化学治疗药物的联合交付有望实现对耐药性肿瘤的有效抑制。在本文中,选择具有高达〜60的耐药指数的高度紫杉醇(PTX)耐药性卵巢癌(OV),以评估有效的基因/药物共递送纳米载体的性能。受细胞和外泌体中发生的自组装的启发,我们设计了直径约为100 nm的仿生脂质/葡聚糖杂化纳米载体,以增强细胞内吞作用和药物/基因释放的效率。该纳米载体是通过脂质两亲物在氧化还原可切割的葡聚糖基纳米凝胶表面上的框架引导的自组装而制备的。将阴离子MDR1-siRNA和疏水性药物PTX分别加载到杂化纳米载体的阳离子脂质壳和疏水性内核中。MDR1-siRNA可以敲低MDR1,促进细胞内PTX的积累,因此有望实现对高度PTX耐药的癌细胞的有效抑制作用。体外和体内研究均表明,与仅使用PTX的系统相比,这种双重递送系统显着增强了治疗效果。因此,基因/化学共递送脂质/葡聚糖纳米载体的构建提供了在体外和体内抑制高度耐药性肿瘤的新策略。此外,这项工作将有助于开发迫切需要的肿瘤纳米疗法,该疗法能够克服耐药性,同时还提供无与伦比的有效治疗纳米载体。意义声明:仿生脂质/葡聚糖杂化纳米载体,直径约100 nm,是通过脂质两亲物的框架引导自组装在氧化还原可切割的葡聚糖基纳米凝胶表面上制备的,提供了共同提供MDR1-siRNA和PTX的模型载体。MDR1-siRNA / PTX共同负载的仿生脂质/葡聚糖杂合纳米载体在体外和体内均显示出在高化学抗性人卵巢癌(OV)癌细胞中克服PTX抗性的良好能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号