Redox Biology ( IF 10.7 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.redox.2019.101405 Hyun-Ju Cho 1 , Christopher Harry Switzer 1 , Alisa Kamynina 1 , Rebecca Charles 1 , Olena Rudyk 2 , Tony Ng 3 , Joseph Robert Burgoyne 2 , Philip Eaton 1
|
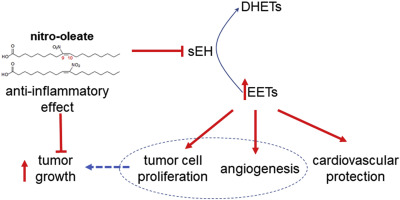
Nitro-oleate (10-nitro-octadec-9-enoic acid), which inhibits soluble epoxide hydrolase (sEH) by covalently adducting to C521, increases the abundance of epoxyeicosatrienoic acids (EETs) that can be health promoting, for example by lowering blood pressure or their anti-inflammatory actions. However, perhaps consistent with their impact on angiogenesis, increases in EETs may exacerbate progression of some cancers. To assess this, Lewis lung carcinoma (LLc1) cells were exposed to oleate or nitro-oleate, with the latter inhibiting the hydrolase and increasing their proliferation and migration in vitro. The enhanced proliferation induced by nitro-oleate was EET-dependent, being attenuated by the ETT-receptor antagonist 14,15-EE-5(Z)-E. LLc1 cells were engineered to stably overexpress wild-type or C521S sEH, with the latter exhibiting resistance to nitro-oleate-dependent hydrolase inhibition and the associated stimulation of tumor growth in vitro or in vivo. Nitro-oleate also increased migration in endothelial cells isolated from wild-type (WT) mice, but not those from C521S sEH knock-in (KI) transgenic mice genetically modified to render the hydrolase electrophile-resistant. These observations were consistent with nitro-oleate promoting cancer progression, and so the impact of this electrophile was examined in vivo again, but this time comparing growth of LLc1 cells expressing constitutive levels of wild-type hydrolase when implanted into WT or KI mice. Nitro-oleate inhibited tumor sEH (P < 0.05), with a trend for elevated plasma 11(12)-EET/DHET and 8(9)EET/DHET (dihydroxyeicosatrienoic acid) ratios when administered to WT, but not KI, mice. Although in vitro studies with LLc1 cells supported a role for nitro-oleate in cancer cell proliferation, it failed to significantly stimulate tumor growth in WT mice implanted with the same LLc1 cells in vivo, perhaps due to its well-established anti-inflammatory actions. Indeed, pro-inflammatory cytokines were significantly down-regulated in nitro-oleate treated WT mice, potentially countering any impact of the concomitant inhibition of sEH.
中文翻译:

可溶性环氧化物水解酶的硝基烯烃依赖性抑制、炎症和肿瘤生长之间的复杂相互关系。
Nitro-oleate (10-nitro-octadec-9-enoic acid) 通过与 C521 共价加成来抑制可溶性环氧化物水解酶 (sEH),增加可促进健康的环氧二十碳三烯酸 (EET) 的丰度,例如通过降低血液压力或它们的抗炎作用。然而,也许与它们对血管生成的影响一致,EET 的增加可能会加剧某些癌症的进展。为了评估这一点,将 Lewis 肺癌 (LLc1) 细胞暴露于油酸或硝基油酸,后者抑制水解酶并增加其体外增殖和迁移. 由硝基油酸诱导的增强增殖是 EET 依赖性的,被 ETT 受体拮抗剂 14,15-EE-5(Z)-E 减弱。LLc1 细胞被设计为稳定过表达野生型或 C521S sEH,后者在体外或体内表现出对硝基油酸依赖性水解酶抑制和相关肿瘤生长刺激的抗性。Nitro-oleate 还增加了从野生型 (WT) 小鼠中分离的内皮细胞的迁移,但对来自 C521S sEH 敲入 (KI) 转基因小鼠的内皮细胞没有增加迁移,这些转基因小鼠经过基因改造以呈现水解酶亲电体抗性。这些观察结果与促进癌症进展的硝基油酸盐一致,因此在体内检查了这种亲电试剂的影响再一次,但这次比较了 LLc1 细胞在植入 WT 或 KI 小鼠时表达组成型野生型水解酶的生长情况。Nitro-oleate 抑制肿瘤 sEH (P < 0.05),当给予 WT 而不是 KI 小鼠时,血浆 11(12)-EET/DHET 和 8(9)EET/DHET(二羟基二十碳三烯酸)比率有升高的趋势。尽管LLc1 细胞的体外研究支持硝基油酸在癌细胞增殖中的作用,但它未能显着刺激体内植入相同 LLc1 细胞的 WT 小鼠的肿瘤生长,这可能是由于其公认的抗炎作用。事实上,在硝基油酸处理的 WT 小鼠中,促炎细胞因子显着下调,可能抵消伴随抑制 sEH 的任何影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号