当前位置:
X-MOL 学术
›
Adv. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption layer formation in dispersions of protein aggregates.
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.cis.2019.102086 B.A. Noskov , A.G. Bykov , G. Gochev , S.-Y. Lin , G. Loglio , R. Miller , O.Y. Milyaeva
Advances in Colloid and Interface Science ( IF 15.9 ) Pub Date : 2019-12-14 , DOI: 10.1016/j.cis.2019.102086 B.A. Noskov , A.G. Bykov , G. Gochev , S.-Y. Lin , G. Loglio , R. Miller , O.Y. Milyaeva
|
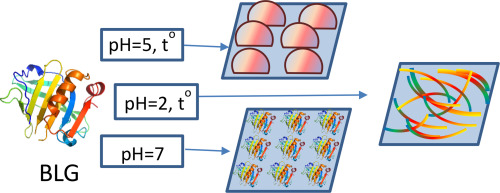
The review discusses recent results on the adsorption of amyloid fibrils and protein microgels at liquid/fluid interfaces. The application of the shear and dilational surface rheology, atomic force microscopy and passive particle probe tracking allowed for elucidating characteristic features of the protein aggregate adsorption while some proposed hypothesis still must be examined by special methods for structural characterization. Although the distinctions of the shear surface properties of dispersions of protein aggregates from the properties of native protein solutions are higher than the corresponding distinctions of the dilational surface properties, the latter ones give a possibility to obtain new information on the formation of fibril aggregates at the water/air interface. Only the adsorption of BLG microgels and fibrils was studied in some details. The kinetic dependencies of the dynamic surface tension and dilational surface elasticity for aqueous dispersions of protein globules, protein microgels and purified fibrils are similar if the system does not contain flexible macromolecules or flexible protein fragments. In the opposite case the kinetic dependencies of the dynamic surface elasticity can be non-monotonic. The solution pH influences strongly the dynamic surface properties of the dispersions of protein aggregates indicating that the adsorption kinetics is controlled by an electrostatic adsorption barrier if the pH deviates from the isoelectric point. A special section of the review considers the possibility to apply kinetic models of nanoparticle adsorption to the adsorption of protein aggregates.
中文翻译:

蛋白质聚集体分散体中的吸附层形成。
该评论讨论了淀粉样原纤维和蛋白质微凝胶在液体/流体界面上的吸附的最新结果。剪切和膨胀表面流变学,原子力显微镜和被动粒子探针跟踪技术的应用可以阐明蛋白质聚集体吸附的特征,而某些提出的假设仍必须通过特殊的方法进行结构表征。尽管蛋白质聚集体分散体的剪切表面性质与天然蛋白质溶液性质的区别高于相应的扩张表面性质的区别,但后者却提供了获得有关在原位形成原纤维聚集体的新信息的可能性。水/空气接口。仅详细研究了BLG微凝胶和原纤维的吸附。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性质,表明如果pH值偏离等电点,则吸附动力学受静电吸附屏障的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性质,表明如果pH值偏离等电点,则吸附动力学受静电吸附屏障的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性能,表明如果pH值偏离等电点,则吸附动力学受静电吸附壁垒的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。
更新日期:2019-12-17
中文翻译:

蛋白质聚集体分散体中的吸附层形成。
该评论讨论了淀粉样原纤维和蛋白质微凝胶在液体/流体界面上的吸附的最新结果。剪切和膨胀表面流变学,原子力显微镜和被动粒子探针跟踪技术的应用可以阐明蛋白质聚集体吸附的特征,而某些提出的假设仍必须通过特殊的方法进行结构表征。尽管蛋白质聚集体分散体的剪切表面性质与天然蛋白质溶液性质的区别高于相应的扩张表面性质的区别,但后者却提供了获得有关在原位形成原纤维聚集体的新信息的可能性。水/空气接口。仅详细研究了BLG微凝胶和原纤维的吸附。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性质,表明如果pH值偏离等电点,则吸附动力学受静电吸附屏障的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性质,表明如果pH值偏离等电点,则吸附动力学受静电吸附屏障的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。如果系统不包含柔性大分子或柔性蛋白片段,则蛋白球,蛋白微凝胶和纯化原纤维的水分散液的动态表面张力和膨胀表面弹性的动力学相关性相似。在相反的情况下,动态表面弹性的动力学相关性可以是非单调的。溶液的pH值强烈影响蛋白质聚集体分散体的动态表面性能,表明如果pH值偏离等电点,则吸附动力学受静电吸附壁垒的控制。该综述的一个特殊部分考虑了将纳米颗粒吸附动力学模型应用于蛋白质聚集体吸附的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号