当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, antileishmanial, and antifungal biological evaluation of novel 3,5‐disubstituted isoxazole compounds based on 5‐nitrofuran scaffolds
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-12-16 , DOI: 10.1002/ardp.201900241 Ozildéia S Trefzger 1 , Natália V Barbosa 1, 2 , Renata L Scapolatempo 1 , Amarith R das Neves 1, 2 , Maria L F S Ortale 1 , Diego B Carvalho 1 , Antônio M Honorato 2 , Mariana R Fragoso 1 , Cristiane Y K Shuiguemoto 1 , Renata T Perdomo 3 , Maria F C Matos 3 , Marilene R Chang 4 , Carla C P Arruda 2 , Adriano C M Baroni 1
Archiv der Pharmazie ( IF 4.3 ) Pub Date : 2019-12-16 , DOI: 10.1002/ardp.201900241 Ozildéia S Trefzger 1 , Natália V Barbosa 1, 2 , Renata L Scapolatempo 1 , Amarith R das Neves 1, 2 , Maria L F S Ortale 1 , Diego B Carvalho 1 , Antônio M Honorato 2 , Mariana R Fragoso 1 , Cristiane Y K Shuiguemoto 1 , Renata T Perdomo 3 , Maria F C Matos 3 , Marilene R Chang 4 , Carla C P Arruda 2 , Adriano C M Baroni 1
Affiliation
|
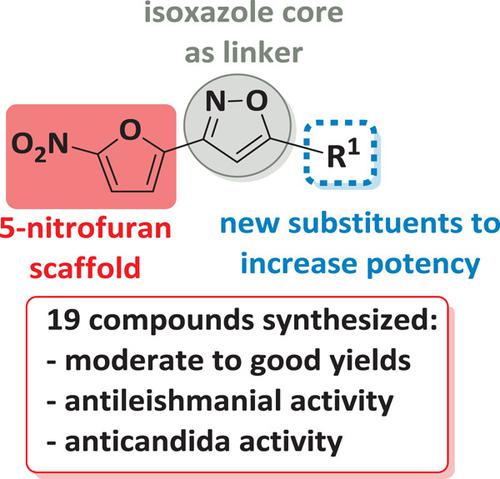
Nineteen 3,5‐disubstituted‐isoxazole analogs were synthesized based on nitrofuran scaffolds, by a [3 + 2] cycloaddition reaction between terminal acetylenes and 5‐nitrofuran chloro‐oxime. The compounds were obtained in moderate to very good yields (45–91%). The antileishmanial activity was assayed against the promastigote and amastigote forms of Leishmania (Leishmania) amazonensis. Alkylchlorinated compounds 14p–r were active on both the promastigote and amastigote forms, with emphasis on compound 14p, which showed strong activity against the amastigote form (IC50 = 0.6 μM and selectivity index [SI] = 5.2). In the alkyl series, compound 14o stands out with an IC50 = 8.5 μM and SI = 8.0 on the amastigote form. In the aromatic series, the most active compounds were those containing electron‐donor groups, such as trimethoxy isoxazole 14g (IC50 = 1.2 μM and SI = 20.2); compound 14h, with IC50 = 7.0 μM and SI = 6.1; and compound 14j containing the 4‐SCH3 group, with IC50 = 5.7 μM and SI = 10.2. In addition, the antifungal activity of 19 nitrofuran isoxazoles was evaluated against five strains of Candida (C. albicans, C. parapsilosis, C. krusei, C. tropicalis, and C. glabrata). Eleven isoxazole derivatives were active against C. parapsilosis, and compound 14o was found to be the most active (minimal inhibitory concentration [MIC] = 3.4 μM) for this strain. Compound 14p was active against all the strains tested, with an MIC = 17.5 μM for C. glabrata, lower than that of the fluconazole used as the reference drug.
中文翻译:

基于 5-硝基呋喃支架的新型 3,5-二取代异恶唑化合物的设计、合成、抗利什曼原虫和抗真菌生物学评价
基于硝基呋喃支架,通过末端乙炔和 5-硝基呋喃氯肟之间的 [3 + 2] 环加成反应合成了 19 种 3,5-二取代的异恶唑类似物。以中等至非常好的产率 (45–91%) 获得这些化合物。针对前鞭毛体和无鞭毛体形式的利什曼原虫 (Leishmania) amazonensis 测定了抗利什曼原虫活性。烷基氯化化合物 14p–r 对前鞭毛体和无鞭毛体形式均有活性,重点是化合物 14p,它对无鞭毛体形式表现出很强的活性(IC50 = 0.6 μM 和选择性指数 [SI] = 5.2)。在烷基系列中,化合物 14o 以无鞭毛体形式的 IC50 = 8.5 μM 和 SI = 8.0 脱颖而出。在芳香族系列中,最活跃的化合物是那些含有电子给体基团的化合物,如三甲氧基异恶唑 14g (IC50 = 1. 2 μM 和 SI = 20.2);化合物 14 小时,IC50 = 7.0 μM,SI = 6.1;以及含有 4-SCH3 基团的化合物 14j,IC50 = 5.7 μM,SI = 10.2。此外,还评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。
更新日期:2019-12-16
中文翻译:

基于 5-硝基呋喃支架的新型 3,5-二取代异恶唑化合物的设计、合成、抗利什曼原虫和抗真菌生物学评价
基于硝基呋喃支架,通过末端乙炔和 5-硝基呋喃氯肟之间的 [3 + 2] 环加成反应合成了 19 种 3,5-二取代的异恶唑类似物。以中等至非常好的产率 (45–91%) 获得这些化合物。针对前鞭毛体和无鞭毛体形式的利什曼原虫 (Leishmania) amazonensis 测定了抗利什曼原虫活性。烷基氯化化合物 14p–r 对前鞭毛体和无鞭毛体形式均有活性,重点是化合物 14p,它对无鞭毛体形式表现出很强的活性(IC50 = 0.6 μM 和选择性指数 [SI] = 5.2)。在烷基系列中,化合物 14o 以无鞭毛体形式的 IC50 = 8.5 μM 和 SI = 8.0 脱颖而出。在芳香族系列中,最活跃的化合物是那些含有电子给体基团的化合物,如三甲氧基异恶唑 14g (IC50 = 1. 2 μM 和 SI = 20.2);化合物 14 小时,IC50 = 7.0 μM,SI = 6.1;以及含有 4-SCH3 基团的化合物 14j,IC50 = 5.7 μM,SI = 10.2。此外,还评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。评估了 19 种硝基呋喃异恶唑对五种念珠菌菌株(白色念珠菌、近平滑念珠菌、克柔念珠菌、热带念珠菌和光滑念珠菌)的抗真菌活性。十一种异恶唑衍生物对近平滑念珠菌具有活性,并且发现化合物 14o 对该菌株的活性最强(最小抑制浓度 [MIC] = 3.4 μM)。化合物 14p 对所有测试菌株均有活性,对光滑念珠菌的 MIC = 17.5 μM,低于用作参考药物的氟康唑。











































 京公网安备 11010802027423号
京公网安备 11010802027423号